Earth's Heavy Metal Allotment
September 19, 2011
As a
materials scientist, I learned to value the useful properties of
elements of high
atomic number. At one time I had in my
laboratory a quarter of a million dollars worth of
platinum items that were used for preparing
single crystals of
oxides. Platinum's atomic number is 78. Those items not in use were dutifully locked in a
safe that was so secure it would take me several tries to set the
combination lock to open.
All my platinum items were dutifully
inventoried each month as a precaution. One nearby manufacturer had several platinum
crucibles stolen when the plant was closed because of a
blizzard. As I remember, the theft was an "
inside job," and the culprit was easily apprehended. It would be very hard to convert platinum to money undetected.
Unfortunately, the way that the
universe creates elements via
nucleosynthesis in
stars favors low atomic numbers. That's why the
Earth is mostly composed of
minerals like
silicates of
lithium (atomic number 3),
sodium (atomic number 11),
magnesium (atomic number 12), and
aluminum (atomic number 13), with a touch of
iron (atomic number 26).
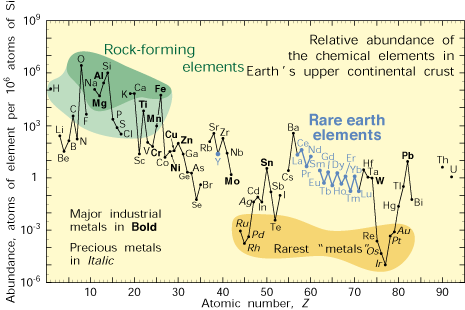
Abundance of the chemical elements in Earth's upper continental crust as a function of atomic number.
(USGS)
Although the heavy elements may be rare in the Earth's
crust, they should actually be rarer still. As the Earth formed about 4.5 billion years ago,[1] its iron content settled into the
core, pulling most of the heavy elements with it. Platinum, for example, is very soluble in iron.[2] Our
technological age would be a much more barren place if we were forced to live with Earth's initial crustal allotment of elements such as platinum,
gold and
tungsten.
As it turns out, the concentration of such elements in the crust is about four
orders of magnitude higher than they should be. The working theory for this has always been that a
meteor bombardment, called the
Late Heavy Bombardment, seeded the crust with these useful elements. This was the same bombardment that
cratered the
moon. But how do you prove such a
theory? You
analyze rocks that are nearly four billion years old.[3-5]

(Drawing by Tim Wether,
via Wikimedia Commons)
Matthias Willbold and
Tim Elliott of the
University of Bristol did an
isotopic analysis of elements in 3.8 billion-year-old rocks from
Isua, Greenland. These rocks were collected by
Stephen Moorbath of
Oxford University.[5] The focus of the study was tungsten, which is present in these rocks at just ten
parts per million.[5]
What they observed was a thirteen parts per million difference in the ratio of the isotopes,
182W/
184W, between the 3.8 million year old rocks and present day rocks.[3] The old rocks had the higher ratio. As can be imagined, the analysis of such small quantities is exceedingly difficult.
Said Willbold,
"Extracting tungsten from the rock samples and analyzing its isotopic composition to the precision required was extremely demanding given the small amount of tungsten available in rocks. In fact, we are the first laboratory world-wide that has successfully made such high-quality measurements."[5]
The measurement is consistent with the late heavy bombardment being the source of the heavy elements, and it excludes other scenarios, such as partial remixing of a
deep-mantle reservoir.[3]
This research was funded by the
Natural Environment Research Council (NERC), the
Science and Technology Facilities Council (STFC) and the
German Research Foundation (Deutsche Forschungsgemeinschaft, DFG).[5]
References:
- "Age Of The Earth," US Geological Survey, July 9, 2007.
- Platinum-Iron Phase Diagram, PGM Database. See the PGM Database for other phase diagrams.
- Matthias Willbold, Tim Elliott and Stephen Moorbath, "The tungsten isotopic composition of the Earth's mantle before the terminal bombardment," Nature, vol. 477, no. 7363 (September 8, 2011), pp. 195-198.
- Thorsten Kleine, "Geoscience: Earth's patchy late veneer," Nature, vol. 477, no. 7363 (September 8, 2011), pp. 168-169
- "Where does all the gold come from?" Bristol University Press Release, September 7, 2011.
- This Island Earth (1955), Joseph M. Newman, Director, on the Internet Movie Database.
Permanent Link to this article
Linked Keywords: Materials scientist; elements; atomic number; laboratory; platinum; single crystal; oxide; safe; combination lock; inventory; crucible; Northeastern United States blizzard of 1978; inside job; universe; nucleosynthesis; star; Earth; mineral; silicate; lithium; sodium; magnesium; aluminum; iron; USGS; crust; core; technology; gold; tungsten; order of magnitude; meteor; Late Heavy Bombardment; crater; moon; theory; analysis; This Island Earth; Wikimedia Commons; Matthias Willbold; Tim Elliott; University of Bristol; isotope; Isua, Greenland; Stephen Moorbath; Oxford University; parts per million; deep-mantle; Natural Environment Research Council; Science and Technology Facilities Council; German Research Foundation; US Geological Survey; PGM Database.