Flash Bainite
June 29, 2011
What could be scientifically exciting about
steel, especially to someone with a
physics background? I once remarked that a
physicist is the type of
materials scientist who tries to find steel in the
periodic table. A physicist is also the type of
computer scientist who writes a
database in
Fortran. I'm guilty on both counts, although in my case, I used
Visual Basic instead of Fortran. I even wrote a
web browser in Visual Basic. It operated on local server files and used my own
hypertext markup language before "http" and ubiquitous use of the
Internet splashed onto the scene.
Graduate students discover that much of their education is tied to the special interests of their
professors. When you've worked on a topic for many years, sometimes to the exclusion of all others, it starts to seem like the most important thing in the world. So, when a professor teaches a course, he's likely to return to his pet topic, again and again. That's how I became an expert in
Ostwald ripening, as I discussed in a
previous article (Ostwald Ripening, March 23, 2007).
For the same reason, I got to know quite a bit about the
Martensitic transformation, a
diffusionless transformation that's important in the manufacture of steel. This type of transformation from one
crystal structure to another involves just the slightest rearrangement of
atoms, usually less than the
interatomic spacing, and that's why it's termed diffusionless.
The Martensitic transformation is responsible for the
shape-memory effect in certain alloys, notably
Ni55Ti45. If you gently bend a piece of this Martensitic material at
room temperature, it will retain the bent shape. Heating the piece above a particular temperature will transform the
Martensite to
austenite, the piece will revert to its original shape, and it will keep that shape even when cooled again to room temperature, forming Martensite. This (austenite
<--> Martensite) transformation is reversible for NiTi, but it is not reversible in steel. Steel is not a shape-memory alloy.
In steelmaking,
Martensite is a
body-centered tetragonal crystal phase of
iron and
carbon. It's produced by cooling from austenite, a
face-centered cubic phase. The presence of Martensite makes steel
harder. Martensite is not an
equilibrium phase of the iron-carbon system, so it can't be found in the
equilibrium phase diagram. This phase transition has a very small
activation energy, since it involves such a slight movement of carbon atoms, so it can even occur at
cryogenic temperatures in some materials.
Mechanical stress can cause the transformation, also.
Bainite is another non-equilibrium microstructure formed from austenite that produces a hardened steel. It forms upon rapid cooling through a temperatures range of about 300 - 500 °C, depending on the presence of
alloying elements. Bainite was discovered in the 1920s, but it's seen a renewed interest in a material called Flash Bainite, invented by a small Detroit company,
SFP Works, LLC.[1-5]
The patent pending process[2] for Flash Bainite is claimed to produce steel that's seven percent stronger than Martensitic steel. It involves an extremely rapid heating and cooling cycle; thus, the "flash" appellation. The process has been described in a paper in the journal, Materials Science and Technology,[3] and a schematic diagram of the process appears below.[2]
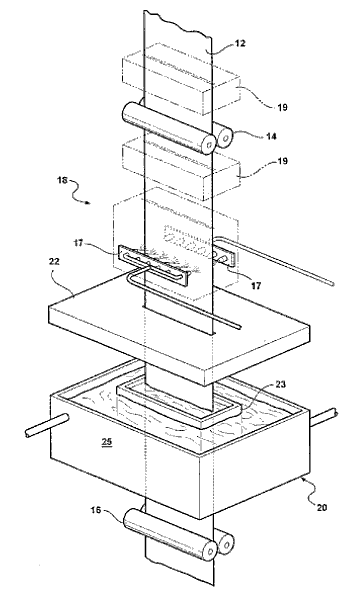
The Flash BainiteTM process, as described in its US patent application, "Method and Apparatus for Micro-Treating Iron-Based Alloy, and the Material Resulting Therefrom," by Gary M. Cola, Jeff W. Ziolkowski and Todd C. Ziolkowski.
The roll steel product is passed quickly through a heating section (17) and a cooling bath (23).
(Figure 2 of US Patent Application 11/718,969, Publication No. US 2007/0261770 A1, November 16, 2005).[2])
An example of the Flash Bainite process, as described in this paper, subjects a sheet of
AISI-8620 steel to a rapid heating and cooling cycle of less than ten seconds duration. The hot zone temperature is about 1100°
C, and the cooling is by a
water quench. The resultant product has a high
ultimate tensile strength (1694
MPa) and a high
elongation (7·1%). This is a 7% higher strength and 30% greater elongation than high strength Martensitic steel (1585 MPa, 5·1%). Microscopy showed a distribution of bainitic and Martensite microstructures with
carbides. As claimed on the company web site, their Flash 4130 product exceeds the strength-to-weight ratio of the
titanium alloy, Ti-64, at just 10% the cost.
Of course, the thermodynamics of the process requires a thin working material, but there are abundant applications in sheet and tubing products. The process is claimed to be environmentally friendly, also. Only a
kilowatt/
kilogram of energy is needed to heat the feedstock, and the cooling is by a water quench, not with
oil or molten
salt.[4]
Gary Cola, the inventor of Flash Bainite is a self-trained
metallurgist, so he tapped into the scientific expertise of
Ohio State University to validate his process.[5] He enlisted the aid of
Suresh Babu, an associate professor of
materials science and engineering at Ohio State, who admits that steelmaking is a "mature technology" with very few surprises.[5] Babu is also Director of the co-located
National Science Foundation Center for Integrative Materials Joining for Energy Applications.
The material properties tested true, and the Ohio State team investigated the mechanism. A typical slow cooling will produce Martensite from an austenitic steel, but the flash process is so rapid that the carbide phase does not dissolve completely during heating, and you end up with a complex microstructure of bainite, Martensite and carbides after cooling.
All this reminds me of a
Design of Experiments course I took many years ago. In a designed experiment, you write down all the possible variables of a process, and then run
experiments at the limits of each variable. If cooling rate and heating rate are two variables, you would do four experiments with (cooling rate, heating rate) set at (high, high), (low, low), (low, high) and (high, low). Flash Bainite would fall into the (high, high) class, but the trick is knowing what's really high, and what's really low. A "skilled" metallurgist would likely never venture into the rates as extreme as those for Flash Bainite.
References:
- G. Cola, Jr., "Temper Resistant 1600mpa / 8.7%El Aisi1020 Flash Bainite Processed Steel Made In 5 Seconds," SFP Works LLC Preprint (SFP Works LLC, 11825 29 Mile Rd, Washington Twp, Michigan USA).
- Gary M. Cola, Jeff W. Ziolkowski and Todd C. Ziolkowski, "Method and Apparatus for Micro-Treating Iron-Based Alloy, and the Material Resulting Therefrom," US Patent Application No. 11/718,969, Publication No. US 2007/0261770 A1, November 16, 2005.
- T. Lolla, G. Cola, B. Narayanan, B. Alexandrov and S.S. Babu, "Development of rapid heating and cooling (flash processing) process to produce advanced high strength steel microstructures," Materials Science and Technology, vol. 27, no. 5, (May, 2011), pp. 863-875. Preprint available at http://www.bainitesteel.com/FlashProcess.pdf
- Bainite Steel Web Site.
- Pam Frost Gorder, "A New Way To Make Lighter, Stronger Steel – In A Flash," Ohio State University Press Release, June 14, 2011.
Permanent Link to this article
Linked Keywords: Steel; physics; physicist; materials scientist; periodic table; computer scientist; database; Fortran; Visual Basic; web browser; hypertext markup language; Internet; Graduate student; professor; Ostwald ripening; Martensitic transformation; diffusionless transformation; crystal structure; atom; interatomic spacing; shape-memory effect; nickel; Ni; titanium; Ti; room temperature; Martensite; austenite; Martensite; body-centered tetragonal; iron; carbon; face-centered cubic; hardness; equilibrium phase; equilibrium phase diagram; activation energy; cryogenic temperature; mechanical stress; Bainite; alloying element; SFP Works, LLC; US Patent Application 11/718,969, Publication No. US 2007/0261770 A1; AISI-8620 steel; Celsius; C; water quench; ultimate tensile strength; MPa; elongation; carbide; titanium; kilowatt; kilogram; oil; salt; metallurgist; Ohio State University; Suresh Babu; materials science and engineering; National Science Foundation Center for Integrative Materials Joining for Energy Applications; Design of Experiments; experiment.