Salt Water Energy
May 20, 2011
In our quest for
energy, we sometimes enter regions of
unintended consequences.
Three Mile Island,
Chernobyl and
Fukushima can be placed in this category, but we needn't stigmatize the
nuclear industry, alone. A recent article in the
Proceedings of the National Academy of Sciences reports how drilling for the
natural gas found in
shale has contaminated some
groundwater supplies.[1]
The noted
environmentalist, Lester B. Lave of
Carnegie Mellon University, who died on May 9, 2011,[2] founded the field of
life-cycle assessment that analyzes the environmental impact of products from "cradle to grave." When we look at an
automobile, we shouldn't focus just on the
tailpipe emissions. We should look also at all the polluting processes that went into its manufacture, such as
mining its
metal and making the many sets of
tires it will need over its lifetime. There's also the problem of its final disposition, perhaps in a
landfill.
The same type of
environmental audit should be done for
renewable energy devices. When you make your
photovoltaics from
cadmium or
selenium, how does the decommissioning expense figure into your energy cost? More importantly, does the energy needed to manufacture your renewable energy device exceed the energy you'll get from it? There are also the costs involved in siting and installing your device, and its subsequent maintenance. The reason that most photovoltaics are on roofs is that land is generally too expensive to have a
solar farm as its sole occupant.
In 2009,
Doriano Brogioli of the
Università degli Studi di Milano-Bicocca,
Monza, Italy, published the idea of an interesting energy generator in
Physical Review Letters.[3] His device was a
supercapacitor that's alternatively exposed to
salt water and freshwater. Brogioli's effect is like that observed in a common
physics experiment in which a
capacitor is charged at a certain
voltage, and then its
dielectric is removed. When this happens, the voltage on the capacitor plates increases.
The reason for this is that the voltage
V on a capacitor is given as the ratio of its stored
charge Q and its capacitance
C; namely
V = Q/C
Since the charge
Q is stored on the conducting plates, the charge is unchanged when the dielectric is removed. Since the capacitance is decreased through removal of the dielectric, the voltage increases. More importantly, the energy stored in the capacitor increases. The stored energy of a capacitor
U is given by
U = (1/2) C V2
Since we're always mindful of
conservation laws (in this case, physical, not environmental), where does this extra energy come from? It comes from the work we did in removing the dielectric. Our capacitor has become a transformer that changes
mechanical work to
electrical power.
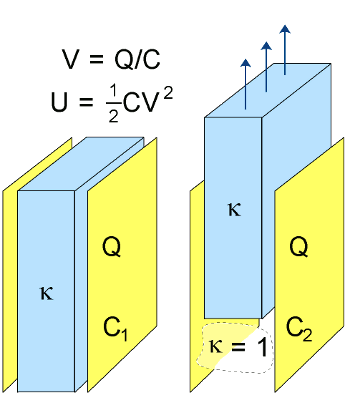
Removal of a dielectric from a parallel plate capacitor.
The charge remains constant, but the plate voltage and stored energy increase.
Illustration by the author, via Wikimedia Commons
The energy we see at the capacitor plates is a function of the
dielectric constant κ and the properties of the capacitor. If the final dielectric is air (κ=1), and the final capacitance is
C, then the energy we get is easily calculated:
ΔU = (1/2) (Q2/C) (κ - 1)
Brogioli's device uses the double-layer capacitance effect found in supercapacitors. Large surface area
carbon electrodes, like those used in supercapacitors, are placed in a
saline solution and the resultant capacitor is charged. Just as in a supercapacitor, it's the
ions in solution that are important. When voltage is applied to the salt water solution, the
sodium and
chlorine ions migrate to their appropriate electrodes. When fresh water is allowed to displace the saline, the capacitor energy increases as the ions diffuse away from the electrodes, increasing the plate voltage.
Just as in the case of withdrawal of a solid dielectric from a parallel plate capacitor, work must be done to remove the ions at the electrodes. That's because the
electrostatic forces try to hold the ions at the electrodes as the
diffusion of fresh water carries them away. Brogioli's small test cell gave just five
microjoules of energy per cycle, but he estimates a potential for 1.6 KJ per
liter of fresh water.[3-4] One immediate problem is that the voltage must be kept below one volt so that
chemical reactions don't take place. There is a similar problem in supercapacitors.
Yi Cui, an Associate Professor of
Materials Science and Engineering at
Stanford University, and his
research team have been extending Brogioli's design using a
cathode of
sodium-manganese dioxide (Na
2−xMn
5O
10)
nanorods that increase the surface area and allow a faster diffusion of the sodium ions in and out of the cathode.[5-7] Unfortunately,
silver is needed as the
anode.
Using
Pacific Ocean seawater and freshwater from
Donner Lake, Cui's team obtained 74% percent of the possible energy that can be obtained from this mechanism. According to Cui, 50 cubic meters of freshwater per second would produce about 100
megawatts of power, which is enough electricity for about 100,000 households.[6]
How much energy is available this way? A 1974 paper by Richard S. Norman[8] estimated that the freshwater runoff of the US alone yields about 10 gigawatts at 25% efficiency. Worldwide, it's as if each river running into the ocean were terminated in a 225
meter waterfall at its mouth.
Of course, everything has its downside. As I wrote in a
previous article (The Water Equivalent of Energy, June 1, 2010), freshwater is becoming a scarce material. This point is reiterated in a recent book review in
Nature.[9] According to the
World Bank,
India's supplies of fresh water will be exhausted by 2050; and
China, which has
polluted about seventy percent of its water supplies, will run out of fresh water by 2030. Mixing freshwater with salt water might not be a good idea in the long run.
References:
- Stephen G. Osborn, Avner Vengosh, Nathaniel R. Warner andRobert B. Jackson, "Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing," Proc. Natl. Acad. Sci., Published online before print, May 9, 2011, doi: 10.1073/pnas.1100682108.
- Dan Majors, "Obituary: Lester B. Lave / Carnegie Mellon economics professor and visionary researcher," Pittsburgh Post-Gazette, May 10, 2011.
- Doriano Brogioli, "Extracting Renewable Energy from a Salinity Difference Using a Capacitor," Phys. Rev. Lett. vol. 103, no. 5 (July 31, 2009).
- Lauren Schenkman, "Buzz Blog: Electricity From Salty Water," Physics Central, July 23, 2009.
- Fabio La Mantia, Mauro Pasta, Heather D. Deshazer, Bruce E. Logan and Yi Cui, "Batteries for Efficient Energy Extraction from a Water Salinity Difference," Nano Lett., vol. 11, no. 4 (March 17, 2011), pp 1810-1813.
- Louis Bergeron, "Stanford researchers use river water and salty ocean water to generate electricity," Stanford Report, March 28, 2011
- Dexter Johnson, "Nanomaterial Boosts Efficiency of Salinity Power Technology," IEEE Spectrum, May 06, 2011.
- Richard S. Norman, "Water Salination: A Source of Energy," Science, Vol. 186 no. 4161 (October 25, 1974), pp. 350-352.
- Margaret Catley-Carlson, "Environment: Water, water everywhere...," Nature, vol. 473, no. 7345 (May 5, 2011), pp.27-28.
Permanent Link to this article
Linked Keywords: Energy; unintended consequences; Three Mile Island; Chernobyl; Fukushima; nuclear industry; Proceedings of the National Academy of Sciences; natural gas; oil shale; groundwater; environmentalist; Carnegie Mellon University; life-cycle assessment; automobile; tailpipe emissions; mining; metal; tire; landfill; environmental audit; renewable energy; photovoltaic; cadmium; selenium; solar farm; Doriano Brogioli; Università degli Studi di Milano-Bicocca; Monza, Italy; Physical Review Letters; supercapacitor; salt water; physics; capacitor; voltage; dielectric; charge; conservation law; mechanical work; electrical power; Wikimedia Commons; relative permittivity; dielectric constant; carbon; electrode; saline solution; ion; sodium; chlorine; electrostatic force; diffusion; microjoule; liter; chemical reaction; Yi Cui; Materials Science and Engineering; Stanford University; cathode; sodium-manganese dioxide; nanorod; silver; anode; Pacific Ocean; Donner Lake; megawatt; meter; waterfall; Nature; World Bank; India; China; water pollution.