Atmospheric Dust
February 2, 2011
Fundamental studies, when first undertaken, often seem superfluous to outside observers. Experiments at
Rutherford's Cambridge University Cavendish laboratory a hundred years ago must have seemed to be as useless and esoteric as the
number theory of fellow Cambridgian,
Godfrey Harold "G. H." Hardy. Hardy left Cambridge in 1919, the year that Rutherford arrived. Hardy was proud to state (in what might be a
paraphrase of a longer quotation),
"Nothing I have ever done is of the slightest practical use."
Research at Rutherford's Cavendish laboratory included the existence of the
neutron and the discovery of the
isotopes of the
elements, fundamental science that has had significant technological fallout.[1] It now appears that some fundamental studies of the way that
brittle materials fracture may lead to an important correction in our understanding of the affect of atmospheric dust on
insolation.
Material
fracture studies were pioneered by
Alan Arnold Griffith of the eponymous
Griffith's criterion (or law). Griffith's law states that the
ultimate strength of a brittle solid is inversely proportional to the square root of the largest flaw size. Griffith published his fracture study in 1921, and much fundamental work on the fracture of brittle materials has been done in the ninety years since. One important result is that fragmentation of brittle materials, at least in the regime in which they actually do fracture, is
scale invariant; that is, smaller bodies break apart just like larger bodies. Large items, such as drinking glasses, and small dust particles break apart in the same way. In general, the particle size distribution of fracture pieces follows the
power law,
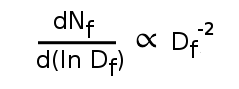
where N
f is the number of fragments with size D
f.
It's not surprising that atmospheric dust has a big impact on the amount of sunlight that reaches the ground. Jasper F. Kok of the
National Center for Atmospheric Research,
Boulder, Colorado, has just published a paper in the
Proceedings of the National Academy of Sciences [2-4] that demonstrates that the scale-invariant fragmentation of brittle materials is applicable to dust that's emitted into the atmosphere. The result is that current
global circulation models overestimate the fraction of
clay particles of size less than two
micrometers in diameter in the atmosphere by a factor of 2-8. As a result, there are many more dust particles in the atmosphere than previously thought, since the fractured dust particles produce many larger fragments. These errors have led to an overestimate of the
radiative cooling of a given quantity of emitted dust.
Dust particles in the atmosphere range in size from as large as 50 micrometers down to 100
nanometers in diameter. The larger particles, defined as
silt particles, remain aloft for a few days; but smaller, two micrometer-sized, clay, particles will remain for about a week. Because of their long residence time, the clay particles will encircle much of the Earth, reflecting heat back into space. The present global circulation models assume a far greater fraction of heat-reflecting smaller particles than are actually present. The PNAS paper says that the ratio of the larger silt particles to the smaller clay particles is two to eight times greater than previously thought.
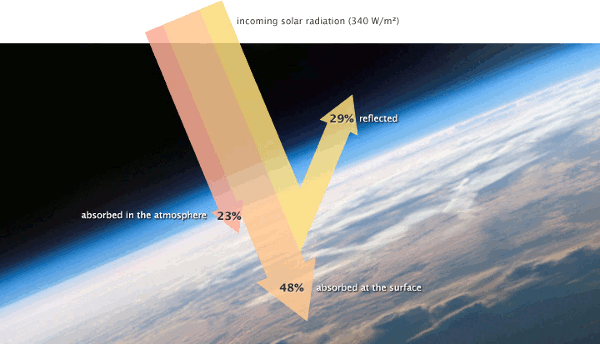
Affect of atmospheric dust on Earth's insolation.
(National Center for Atmospheric Research)
Aside from the apparent implications for
weather forecasting in dusty regions, there are other, subtle effects in Earth's
ecosystem. The oceans may receive more
iron from airborne particles, which will have a fertilizing effect on marine
flora. Growth of marine plant matter is a
carbon dioxide sink. Dust also falls on mountain
snowpacks, which accelerates the melting of snow.
As for the useless and esoteric number theory - It would be a surprise to Hardy that number theory has actually become useful to such things as
public key cryptography.
References:
- Pun intended, but probably in bad taste.
- "Broken glass yields clues to climate change," University Corporation for Atmospheric Research News Center, December 27, 2010.
- Cheryl Dybas and David Hosansky, "Broken Glass Yields Clues to Climate Change," National Science Foundation Press Release 10-243, December 27, 2010.
- Jasper F. Kok, "A scaling theory for the size distribution of emitted dust aerosols suggests climate models underestimate the size of the global dust cycle," Proc. Natl. Acad. Sci., vol. 108, no. 3 (January 18, 2011), pp. 1016-1021.
Permanent Link to this article
Linked Keywords: Ernest Rutherford; Cambridge University; Cavendish laboratory; number theory; Godfrey Harold Hardy; G. H. Hardy; neutron; isotope; chemical elements; brittle materials; fracture; insolation; fracture studies; Alan Arnold Griffith; Griffith's criterion; ultimate strength; scale invariance; power law; National Center for Atmospheric Research; Boulder, Colorado; Proceedings of the National Academy of Sciences; global circulation model; clay; micrometer; radiative cooling; nanometer; silt; weather forecasting; ecosystem; iron; flora; carbon dioxide; snowpack; public key cryptography.