Combinatorial Research
December 12, 2011
In a
previous article (Tea Party Technologists, November 18, 2011), I used my power of the pen (now, more like the keyboard) to rant about the unstructured approaches to
experiment that eschew
theory and take a
shotgun-like approach to
innovation. This problem is not new. At the time that
Fermi was making a mark on
Italian physics, he had the unique problem that many Italian
scientists thought that the future lay in unstructured inquiry; that is, try everything and
see what works. They reminisced about things like
Galvani's accidental discovery of twitching
frogs' legs. Fermi and his school believed that experiment should be guided by theory.
Combinatorial chemistry, the idea that you should try many combinations of
reactants or
process conditions, has been with us since the 1990s. It became popular at the same time that its
enabling technologies,
computing and
robotics, became relatively inexpensive. This approach is popular among
pharmaceutical companies, who apparently use it to produce tens of thousands of unique compounds per year.
A recent paper in
Science makes no excuses about this approach, calling it a "Strategy of Accelerated Serendipity."[1] The research team that published this study used
automation to evaluate a large number of
random reactions for the
synthesis of
benzylic amines. Their robotic instrument was capable of performing more than a thousand reactions per day. They successfully discovered a unique reaction pathway for this synthesis.[1-2] Perhaps the end does justify the means.
Fortunately, the science involved here wasn't just the ability to recognize the desired end-product.
Princeton University professor,
David MacMillan, a co-author of the Science paper, explained that
"...Once we understood how it happened, it set us up to design other completely new reactions based upon our understanding of what happened initially... Now, we're applying similar techniques broadly, finding new reactions continually and determining which ones are important.[2]
A related technique was used by a huge research team to discover a material with giant
magnetostriction. Team members were from the
Department of Materials and Science Engineering,
University of Maryland (College Park, Maryland), the
Material Measurement Laboratory,
National Institute of Standards and Technology (Gaithersburg, Maryland), the
Institute of Metal Physics,
Urals Branch of the Academy of Sciences (Ekaterinburg, Russia), the
School of Mechanical, Industrial, and Manufacturing Engineering,
Oregon State University (Corvallis, Oregon), the
Stanford Synchrotron Radiation Lightsource,
SLAC National Accelerator Laboratory (Menlo Park, California), and the
Department of Physics and Astronomy,
Rowan University (Glassboro, New Jersey).[3-4]
Magnetostriction is a useful material property that allows
mechanical actuation in response to a
magnetic field. Unlike
piezoelectric actuation, which requires a
voltage connection to the actuator, magnetostrictive actuators can be operated without a physical connection. Many magnetostrictive
alloys are made from
rare earth elements, so less expensive alloy compositions are always welcome. For this reason, the research team tried to optimize magnetostriction in
iron-
cobalt alloys.
The research team used a unique combinatorial screening technique. They fabricated a large array of
centimeter length
cantilevers on a
silicon wafer and coated these with a varied ratio of cobalt and iron. The cantilevers allowed a simple means of measuring the magnetostriction by their degree of bending in response to a magnetic field. They subjected these alloy-coated cantilevers to two different
heat treatments, including one that involved a rapid
quench in
water.[4] That the test wafers survived such treatment is a testament to the strength of
single-crystal materials.
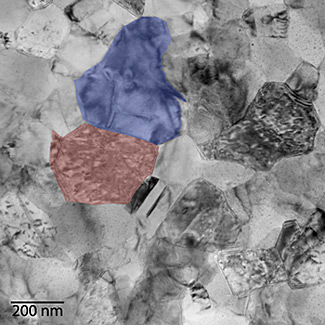
Transmission electron micrograph of an annealed cobalt-iron alloy. The material has a high magnetostriction because of its two-phase structure (iron-rich, blue, and cobalt-rich, red) and the nanoscale segregation.
(Photograph by Bendersky/NIST))
The quenching locked-in a particular
heterogeneous,
nanoscale structure in which cobalt-rich
FCC crystals were embedded in an iron-rich
BCC matrix.[3] The best composition demonstrated considerable magnetostriction in magnetic fields as low as about 100
gauss.[4] For reference, the
Earth's magnetic field is somewhat less than a gauss.
Ichiro Takeuchi, team leader and professor of Materials Science and Engineering at the University of Maryland, says that the magnetostriction of this alloy is less than, but comparable to, the value for
Terfenol-D, a
terbium-
dysprosium-iron alloy with composition TbxDy
1-xFe
2, that's the best magnetostrictive material.[4]
Analysis of the microstructure of the Co
1−xFe
x films indicates that the greatest magnetostriction occurs at compositions near the (fcc+bcc) -> bcc
phase boundary. The desirable properties arise from
precipitation of a cobalt-rich fcc phase in an iron-rich bcc matrix.[3]
References:
- Andrew McNally, Christopher K. Prier and David W. C. MacMillan, "Discovery of an α-Amino C–H Arylation Reaction Using the Strategy of Accelerated Serendipity," Science, vol. 334 no. 6059 (November 25, 2011), pp. 1114-1117.
- Morgan Kelly, "Princeton technique puts chemistry breakthroughs on the fast track," Princeton University Press Release, November 28, 2011.
- Dwight Hunter, Will Osborn, Ke Wang, Nataliya Kazantseva, Jason Hattrick-Simpers, Richard Suchoski, Ryota Takahashi, Marcus L. Young, Apurva Mehta, Leonid A. Bendersky, Sam E. Lofland, Manfred Wuttig and Ichiro Takeuchi, "Giant magnetostriction in annealed Co1−xFex thin-films," Nature Communications, vol. 2, article no. 518, November 1, 2011.
- Michael Baum, "New Magnetic-Field-Sensitive Alloy Could Find Use in Novel Micromechanical Devices," NIST Tech Beat, November 22, 2011.
Permanent Link to this article
Linked Keywords: Experiment; theory; shotgun; innovation; Fermi; Italian; physics; scientist; serendipity; Galvani; frogs' legs; combinatorial chemistry; reagent; reactant; process condition; enabling technology; computing; robotics; pharmaceutical company; science; automation; randomness; random; reaction; synthesis; benzylamine; benzylic amine; Princeton University; David MacMillan; magnetostriction; Department of Materials and Science Engineering; University of Maryland (College Park, Maryland); Material Measurement Laboratory; National Institute of Standards and Technology (Gaithersburg, Maryland); Institute of Metal Physics; Urals Branch of the Academy of Sciences (Ekaterinburg, Russia); School of Mechanical, Industrial, and Manufacturing Engineering; Oregon State University (Corvallis, Oregon); Stanford Synchrotron Radiation Lightsource; SLAC National Accelerator Laboratory (Menlo Park, California); Department of Physics and Astronomy; Rowan University (Glassboro, New Jersey); mechanical actuation; magnetic field; piezoelectric; voltage; alloy; rare earth element; iron; cobalt; centimeter; cantilever; silicon wafer; heat treatment; quench; water; single-crystal material; Bendersky/NIST; heterogeneous; nanoscale; cubic crystal system; face-centered cubic; FCC; body-centered cubic; BCC; gauss; Earth's magnetic field; Ichiro Takeuchi; Terfenol-D; terbium; dysprosium; phase boundary; precipitation.