Coffee Rings (Part II)
August 25, 2011
Scientists will investigate anything that catches their interest, including something as seemingly mundane as
coffee rings. I reviewed the science behind coffee rings in a
previous article (Coffee Rings, December 10, 2010).
Coffee is a
colloid; that is, it's a solution of
solid particles suspended in a
liquid. Coffee rings form because of the outward flow of the suspended particles.
Robert D. Deegan and his colleagues at the
James Franck Institute and the
University of Chicago were the first to investigate coffee rings scientifically, in 1997.[1,2] The Chicago team verified the coffee ring effect also with
red wine,
milk,
tea and
soup. It's been found
experimentally that the size of the suspended particles is important. Colloidal solutions of 100
nm particles will only form rings if the coffee ring is at least 10
μm.[3]

How a materials scientist makes coffee
My trick for a good cup is to grind the beans just before brewing in a drip, or vacuum, coffee maker. The beans are stored in a freezer chest.
(Image via Wikimedia Commons))
Since particle size is important to coffee ring formation, the next question is whether the shape of the particles is important, also. That was the purpose of a study recently published in
Nature by
materials scientists at the
Materials Research Science and Engineering Center at the
University of Pennsylvania.[4-8] Their experiments show that
ellipsoidal particles will not make coffee rings, and their research may have an application to paints and other coatings.
Intuitively, this seems sensible. Coffee rings arise from
capillary fluid flow, caused by
surface tension, outward from the drop's center that pushes the suspended particles to the edge. Ellipsoidal particles would first align with the flow direction, and then allow the fluid to flow around them. This process would leave them in place. However, close experimental examination of the actual process reveals a different mechanism.
The modified flow between the ellipsoidal particles actually draws the particles together to produce loosely packed structures at the
interface between the fluid and air. These structures prevent the suspended particles from independently flowing to the edge to form a ring. The jammed structures deposit where they are formed, and the result is a uniform deposit in the droplet area, and not a ring at the edge. Quite interestingly, adding just a small faction of ellipsoidal particles to a suspension of spheres produces a uniform deposition, also.
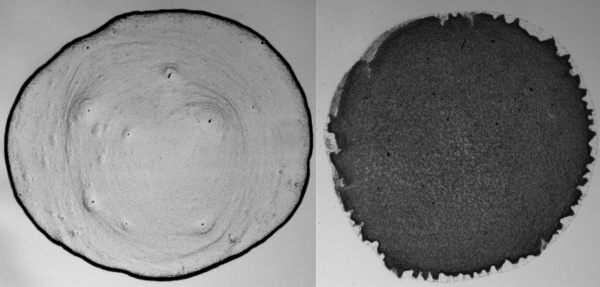
"Coffee stains" when particles are spherical (Left), and ellipsoidal (Right). (Images: Peter J. Yunker and Arjun G. Yodh, University of Pennsylvania, via NSF Web Site). A video demonstration is available on the Internet.[8]
Peter J. Yunker, one of the authors of the Nature paper, has a simple kitchen demonstration of the effect.[6]
"You can observe this effect in a bowl of cheerios. If there are only a few left, they clump together in the middle of the bowl, due to the surface tension of the milk."
Although clumped ellipsoidal particles do reach the edge of the droplet, their packing isn't dense, so there is an even coating of particles when the fluid evaporates.[6]
Surfactants spoil the effect, so adding soap to a colloid of ellipsoidal particles results in the same behavior as one of spherical particles; that is, a ring is formed.[6]
Of course, one good experiment leads to another. The Penn team plans to investigate different particle sizes and shapes, the interplay of mixtures of different particles, and colloids of different types of fluids.[6] When formulating
paints and
inks, control of particle shape allows modification of the deposition process without modifying the
chemistry of the particles or the fluid.[4]
References:
- Robert D. Deegan, Olgica Bakajin, Todd F. Dupont, Greb Huber, Sidney R. Nagel and Thomas A. Witten, "Capillary flow as the cause of ring stains from dried liquid drops," Nature, vol. 389, no. 6653 (23 October 23, 1997), pp. 827-829.
- Diana Steele, "Coffee stains - Fundamental physics revealed in a drop of java," University of Chicago Chronicle, vol. 17, no. 3 (Oct. 23, 1997).
- Xiaoying Shen, Chih-Ming Ho and Tak-Sing Wong, "Minimal Size of Coffee Ring Structure," J. Phys. Chem., vol. B114, no. 16 (March 31, 2010), pp 5269-5274.
- Peter J. Yunker, Tim Still, Matthew A. Lohr and A. G. Yodh, "Suppression of the coffee-ring effect by shape-dependent capillary interactions," Nature, vol. 476, no. 7360 (August 18, 2011), pp. 308-311.
- Jan Vermant, "Fluid mechanics: When shape matters," Nature, vol. 476, no. 7360 (August 18, 2011), pp. 286-287.
- Lisa Van Pay and Evan Lerner, "The Future of Inks, Paints and Coatings Takes Shape," NSF Press Release No. 11-166, August 17, 2011
- Joe Palca, "Scientists Crack The Physics Of Coffee Rings," NPR, August 17, 2011.
- Video explaining how particle shape plays a role in the 'coffee ring effect,' Kurtis Sensenig, University of Pennsylvania, via NSF Web Site.
- Shreyas Mandre, Ning Wu, Joanna Aizenberg and Lakshminarayanan Mahadevan, "Coffee ring deposition in bands," Abstract No. RU.00007 of the 63rd annual meeting of the American Physical Society Division of Fluid Dynamics (Long Beach, CA, November 23, 2010)
- Jason Socrates Bardi, "The physics of coffee rings," American Institute of Physics Press Release, November 23, 2010
- Vladimir A. Belyi, D. Kaya and M. Muthukumar, "Periodic Pattern Formation in Evaporating Drops," arXiv Preprint, December 21, 2006.
- Alvaro G. Marin, Hanneke Gelderblom, Jacco Snoeijer and Detlef Lohse, "Video: Avalanche of particles in evaporating coffee drops, arXiv Preprint, October 15, 2010.
Permanent Link to this article
Linked Keywords: Scientist; coffee ring; coffee; colloid; solid; liquid; Robert D. Deegan; James Franck Institute; University of Chicago; red wine; milk; tea; soup; experiment; nanometer; nm; micrometer; μm; drip; vacuum; Wikimedia Commons; Nature; materials scientist; Materials Research Science and Engineering Center; University of Pennsylvania; ellipsoid; capillary; surface tension; interface; surfactant; paint; ink; chemistry.