Gilbert N. Lewis
November 16, 2011
One hallmark of a good
textbook is that it's been reissued in a revised edition. A book gets even higher marks when it's such a classic that it's revised by another author, many years later. Such was the case for "Thermodynamics," written by the two
physical chemists,
Gilbert N. Lewis and
Merle Randall in 1923. It was revised by
Kenneth S. Pitzer and
Leo Brewer in 1961.[1]
Lewis is best known to
chemistry students for his redefinition of
acids and
bases. A
Lewis acid is an
electron acceptor, and a
Lewis base is an electron donor. As a common example, H
+ is a Lewis acid, and OH
- is a Lewis base. It's never been proven that Lewis exclaimed, "
All your base are belong to us," after making this observation.
Another of Lewis' fundamental contributions to chemistry was the
valence bond theory. In 1916, Lewis proposed that
chemical bonds were shared electrons between atoms that contributed to an enhanced stability for both atoms. This model of the chemical bond was reconciled with
quantum mechanics by
Heitler and
London in 1925, who showed that a merger of
atomic wavefunctions will form a
covalent bond.
Linus Pauling added some detail to the valence bond theory by introducing
resonance, in 1928, and
orbital hybridization, in 1930. This led to publication of his seminal book,
The Nature of the Chemical Bond, in 1939.[2] Lewis' valence bond paper, "The Atom and the Molecule," published in 1916 in the
Journal of the American Chemical Society, was among Pauling's papers.[3]
As they say,
a picture is worth a thousand words, and many a student, myself included, has learned how to bond atoms using the
Lewis dot structures. A similar
pedagogical tool in
physics would be the
Feynman diagram (see figure). On one trip to
Boston for a
scientific conference in the 1980s, I happened to see a Feynman diagram, drawn as graffiti, into a concrete sidewalk. I must have been in
MIT territory. Lewis introduced the
thermodynamic concepts of
activity and
fugacity in a 1908 paper in the Journal of the American Chemical Society.[4]
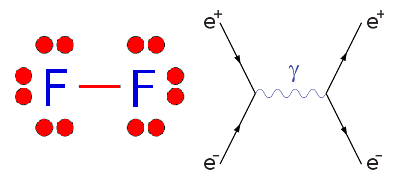
Lewis dot diagram for a fluorine molecule, and the Feynman diagram for electron positron annihilation/pair production. Feynman diagram via Wikimedia Commons)
This year's
Nobel Prizes have been announced, and the announcements give you an idea of the general quality of work that leads to a Nobel Prize. In an assessment of Gilbert N. Lewis's life's work, it's quite apparent that he should have been awarded one. Why wasn't Lewis a
Nobel Laureate? The reason may relate to his argumentative personality.
Lewis had a
post-doctoral fellowship with
Walther Nernst at the
University of Göttingen. While there, Lewis and Nernst had a falling-out. Nernst went on to win the
Nobel Prize in Chemistry in 1920, which made him a qualified nominator for the prize.[5]
However, it appears that the problem was not that Lewis was never nominated. The intrigue goes further. Walther Palmaer, a friend of Nernst, was a member of the selection committee for the chemistry prize. According to references cited on Wikipedia,[6] Palmaer nominated Lewis for the award three times, but he concurrently wrote negative reviews of his work for the prize committee. The committee screens about 200-300 nominations each year, so even a middling review would block the prize.
Not earning the prize must have hurt Lewis all the more deeply when one of his students,
Harold Urey, won the Nobel Prize in Chemistry in 1934 for the discovery of
deuterium. Lewis almost certainly felt he should have shared the prize for his own work on
heavy water. Although Urey had discovered the deuterium isotope of hydrogen in 1931, it was Lewis who prepared the first heavy water specimen in 1933 by
electrolysis.
As another indicator of Lewis' personality, he resigned from the
National Academy of Sciences in 1934, after being a member since 1913. Lewis gave no reason for the resignation, but the resignation appears to have resulted from his general dislike of all the
politics that lurks beneath all human institutions.
Lest we forget,
Richard Feynman also resigned from the Academy. In a resignation letter, dated August 10, 1961, to
Detlev W. Bronk, President of the Academy, Feynman wrote,
"It must be quite a job worrying about all the peculiar whims of all the strange birds that make up your flock... I find it psychologically very distasteful to judge other people's "merit"... To be a member of a group, of which an important activity is to choose others deemed worthy of membership in that self-esteemed group, bothers me."[7]
Lewis was found dead in one of his laboratories in 1946, shortly after a lunch with one of his rivals, and Nobel Chemistry Laureate (1932),
Irving Langmuir. Langmuir was at
Berkeley to receive an
honorary degree. The supposed cause of Lewis' death was a
heart attack. Lewis was seventy,
chain-smoked cigars and didn't watch his diet. However, in the laboratory was broken glassware containing liquid
hydrogen cyanide, so some suspect that the death may have been a suicide.[8]
Langmuir received his Ph.D. in study under Walther Nernst.
References:
- Gilbert Newton Lewis and Merle Randall (Revised by Kenneth S. Pitzer and Leo Brewer, "Thermodynamics (2nd Edition ed.), McGraw-Hill Book Co. (New York, 1961), via Amazon.
- Linus Pauling and The Nature of the Chemical Bond: A Documentary History - Special Collections - Oregon State University
- G.N.Lewis, "The Atom and the Molecule," J.Amer.Chem.Soc. vol. 38, no.4 (April, 1916), pp. 762-785; a copy on the Oregon State University Linus Pauling Web Site.
- G.N.Lewis, "The osmotic pressure of concentrated solutions, and the laws of the perfect solution," J.Amer.Chem.Soc. vol. 30, no.5 (May, 1908), pp. 668-683.
- Nomination and Selection of Chemistry Laureates, Nobel Prize Web Site.
- Gilbert N. Lewis Page on Wikipedia.
- Richard Phillips Feynman and Timothy Ferris, "Perfectly Reasonable Deviations From the Beaten Track: The Letters of Richard P. Feynman," Google eBook.
- Rick DelVecchio,"What Killed Famed Cal Chemist?" San Francisco Chronicle, August 5, 2006.
Permanent Link to this article
Linked Keywords: Textbook; physical chemist; Gilbert N. Lewis; Merle Randall; Kenneth S. Pitzer; Leo Brewer; chemistry; acid; base; Lewis acid; electron; Lewis base; All your base are belong to us; valence bond theory; chemical bond; quantum mechanics; Heitler; London; atomic wavefunction; covalent bond; Linus Pauling; resonance; orbital hybridization; The Nature of the Chemical Bond; Journal of the American Chemical Society; a picture is worth a thousand words; Lewis dot structure; pedagogical tool; physics; Feynman diagram; Boston; scientific conference; Conference on Magnetism and Magnetic Materials; MIT; thermodynamics; activity; fugacity; fluorine; electron positron annihilation; pair production; Wikimedia Commons; Nobel Prize; Nobel Laureate; post-doctoral fellowship; Walther Nernst; University of Göttingen; Nobel Prize in Chemistry; Harold Urey; deuterium; heavy water; electrolysis; National Academy of Sciences; politics; Richard Feynman; Detlev W. Bronk; Irving Langmuir; University of California, Berkeley; honorary degree; heart attack; chain-smoke; cigar; hydrogen cyanide.