| Tikalon Blog is now in archive mode.
An easily printed and saved version of this article, and a link
to a directory of all articles, can be found below: |
|
This article |
| Directory of all articles |
Wiedemann-Franz Law
June 17, 2024
Science is most useful when it makes
predictions. Predictions are based on
theory, and one problem with most theories in
materials science is that they are based on
data that might not exist for all of the intended class of
materials. Early in my
career I developed a theory of how the
concentrations of
elements in
crystals grown from a
molten solution of
lead oxide would depend on their concentrations in that solution.[1] This theory was intended to assist in the
epitaxial growth of
magnetic garnets for
magnetic bubble memories. One
parameter I needed was the
enthalpy of vaporization of some
metal oxides, and many of these had not been
measured.
Fortunately,
Trouton's rule came to my
rescue. This rule, developed by
Irish physicist,
Frederick Trouton (1863-1922), states that the
molar entropy of vaporization ΔSv for most materials is quite close to 85
J/(
mol-
K) at their
boiling points TB.[2] This gave me the ability to
estimate the enthalpy of vaporization
ΔHv from the known boiling points,
ΔHv = TB·ΔSv
While Trouton's rule is valid for many
liquids, it fails when the liquid
components interact peculiarly, as in
hydrogen bonding. That's why the rule fails for
water and other hydrogen bonded materials.

Frederick Trouton, Irish physicist, in 1926.
There have been many important Irish physicists, a few of the most significant are J. D. Bernal (1901-1971), who applied X-ray crystallography to molecular biology; Robert Boyle (1627-1691), famous for Boyle's law; Nicholas Callan (1799-1864), who invented the Induction coil for conversion of low voltages to high-voltage; William Rowan Hamilton (1805-1865), a contributor to mechanics, especially for devising the Hamiltonian; John Joly (1857-1933), who perfected uranium-thorium dating.
Also, Thomas Preston (1860-1900), who discovered the anomalous Zeeman effect; Erwin Schrödinger (1887-1961), who created the eponymous Schrödinger equation; George Stokes (1819-1903), famous for the Navier-Stokes equations; George Stoney (1826-1911), most famous for naming the electron, but he was also the first to estimate the number of gas molecules in a volume; and, John Tyndall (1820-1893), whose research in infrared led to the Tyndall effect, proof of the heat trapping by CO2, and the greenhouse effect.
Wikimedia Commons image from the Proceedings of the Royal Society (London), A110, 4, 1926.
Another
correlation between two material properties is so
precise, in certain limits, that it's known as a
law rather than a
rule. This is the
Wiedemann-Franz law relating
electrical conductivity σ and
thermal conductivity κ; viz.,
κ/σ = LT
in which T is the
absolute temperature, and L is the
proportionality constant, called the
Lorenz number (2.44 x 10
-8 V2-K
-2). The Lorentz number can be given in
fundamental units as
L = (π2/3)·(kB/e)2
in which
kB is the
Boltzmann constant (1.38065 x 10
-23 J-K
-1), and
e is the
elementary charge (1.60218 x 10
-19 coulombs). This law is named after
German physicists,
Gustav Wiedemann (1826-1899) and
Rudolph Franz (1826-1902), who discovered in 1853 that
κ/σ was approximately constant for different metals at the same
temperature.[3]
Danish physicist,
Ludvig Lorenz (1829-1891), deduced the proportionality constant in 1872.
The Wiedemann-Franz law gets its
validity from the fact that in most cases both heat and
electric current are transported by
free electrons in metals. However, electron transport of heat is only dominated by electrons above a certain temperature, called the
Debye temperature, developed by
Peter Debye (1884-1966). Below the Debye temperature, the thermal conductivity of metals decreases more rapidly with increased temperature than the electrical conductivity. Debye also had a
model for the heat capacity that accurately predicts its T
3 behavior at low temperatures, and is known for his work on
X-ray diffraction, for which he was awarded the 1936
Nobel Prize in Chemistry.
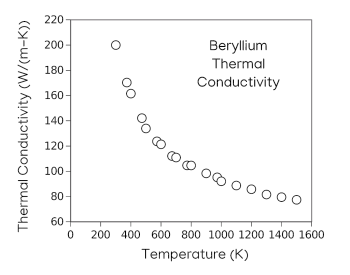
Temperature dependence of the thermal conductivity of beryllium.
Beryllium has the very high Debye temperature of 1440 K (1167 °C), just below its melting point of 1,560 K (1277°C).
This is the usual trend of higher thermal conductivity at lower temperature, just as the trend for electrical conductivity.
(Plotted using Gnumeric from data in ref.4.[4] Click for larger image.)
Measurement of electrical conductivity of
solids and
liquids is somewhat easy, but thermal conductivity measurements are much more difficult. That's the reason that the Wiedemann-Franz law hasn't been tested over an extended range of temperature. A recent
open access paper by
scientists and
engineers from the
University of Virginia (Charlottesville, Virginia), the
European Commission,
Joint Research Centre (Karlsruhe, Germany),
Laser Thermal Analysis, Inc. (Charlottesville, Virginia), and the
University of Rhode Island (Kingston, Rhode Island) describes their
differential radiometry technique used to validate the Wiedemann-Franz law for
tungsten in both its solid and
molten states to temperatures above 2000 K.[5-6] Their paper is published in
Physical Review Letters.[5]
The
research team has made the first direct thermal conductivity measurement for tungsten, the
elemental metal with the highest melting point, 3,695 K (3,422°C).[6] To do this, they used a
laser beam to gradually heat the
center of a 2
millimeter thickness tungsten
disk until it began to melt. The
emitted radiation was
monitored during heating to determine the temperature of the central hot spot. They monitored the required increase in the laser
power required to heat the
area from 2000 K to 4000 K.[6] Data from this technique allowed
calculation of the thermal conductivity for tungsten over this range of temperature using
Fourier's law of thermal conduction.[5-6]
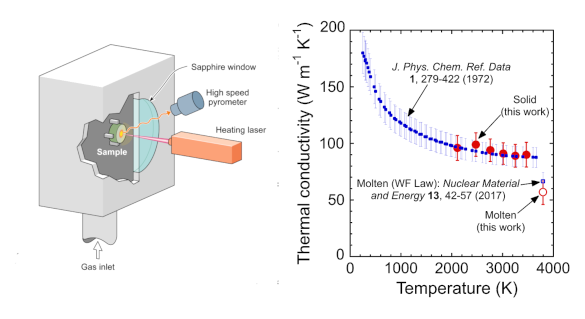
Left, a schematic of the experimental apparatus for the thermal conductivity measurement in which the tungsten disk acts as its own crucible to contain the molten metal at its center. Right, measured thermal conductivity of solid and molten tungsten compared with literature values. (Fig. 1a and fig. 3 of ref. 5,[5] released under the Creative Commons Attribution 4.0 International license. Click for larger image.)
The
technique is a direct measurement of thermal conductivity that avoids the requirement of knowing the material's heat capacity to calculate the thermal conductivity.[5] This single-sided method in which the heat is sourced and temperature is measured from the same side can be used for measurement of thermal conductivity of materials other than tungsten in their molten states.[5] The experiment validated the Wiedemann-Franz law in tungsten even into its molten state.[5]
References:
- D.M. Gualtieri, Flux Growth of (Ca,Ge)-Substituted Rare- Earth Iron Garnets in the Regular Solution Approximation, J. Appl. Phys., vol. 50 (1979), pp. 2170-2172, https://doi.org/10.1063/1.327041. (Surprisingly, the publisher wants $40.00 to read this paper after 45 years!)
- Frederick Trouton, "On Molecular Latent Heat," Philosophical Magazine, vol. 18, no. 110 (1884), pp. 54-57, doi:10.1080/14786448408627563.
- G. Wiedemann and R. Franz, "Ueber die Wärme-Leitungsfähigkeit der Metalle," Annalen der Physik, vol. 165, no. 8 (1853), pp. 497-531, doi:10.1002/andp.18531650802. A free pdf file is available at the same URL.
- Beryllium, ARIES Archive (2013), Office of Fusion Energy Sciences, US Department of Energy.
- Milena Milich, Hunter B. Schonfeld, Konstantinos Boboridis, Davide Robba, Luka Vlahovic, Rudy J. M. Konings, Jeffrey L. Braun, John T. Gaskins, Niraj Bhatt, Ashutosh Giri, and Patrick E. Hopkins, "Validation of the Wiedemann-Franz Law in Solid and Molten Tungsten above 2000 K through Thermal Conductivity Measurements via Steady-State Temperature Differential Radiometry," Phys. Rev. Lett., vol. 132, no. 14 (April 5, 2024), Article no. 146303, DOI:https://doi.org/10.1103/PhysRevLett.132.146303. This is an open access article with a pdf file here.
- Rachel Berkowitz, "Thermal Conductivity Not Too Hot to Handle," Physics, vol. 17, no. 25 (April 5, 2024).
Linked Keywords: Science; prediction in science; scientific theory; materials science; data; material; career; concentration; chemical element; crystal; crystal growth; melting; molten; solution; lead oxide; epitaxy; epitaxial growth; magnetism; magnetic; garnet; magnetic bubble memory; parameter; enthalpy of vaporization; metal; oxide; measurement; measure; Trouton's rule; rescue; Ireland; Irish; physicist; Frederick Trouton (1863-1922); mole (unit); molar; entropy of vaporization; Joule; mol; kelvin; boiling point; approximation; estimate; liquid; component (thermodynamics); interaction; interact; hydrogen bond; hydrogen bonding; water; J. D. Bernal (1901-1971); X-ray crystallography; molecular biology; Robert Boyle (1627-1691); Boyle's law; Nicholas Callan (1799-1864); induction coil; energy transformation; conversion; voltage; William Rowan Hamilton (1805-1865); classical mechanics; Hamiltonian mechanics; Hamiltonian; John Joly (1857-1933); uranium-thorium dating; Thomas Preston (1860-1900); anomalous Zeeman effect; Erwin Schrödinger (1887-1961); eponymous; Schrödinger equation; George Stokes (1819-1903); Navier-Stokes equations; George Stoney (1826-1911); electron; gas molecule; volume; John Tyndall (1820-1893); research; infrared; Tyndall effect; proof (truth); heat; carbon dioxide; CO2; greenhouse effect; Wikimedia Commons; Proceedings of the Royal Society (London); correlation; accuracy and precision; precise; physical law"; rule of inference; Wiedemann-Franz law; electrical conductivity; thermal conductivity; absolute temperature; proportionality constant; Lorenz number; volt; SI base unit; fundamental unit; pi; π; Boltzmann constant; kB; elementary charge; coulomb; Germany; German; Gustav Wiedemann (1826-1899); Rudolph Franz (1826-1902); temperature; Denmark; Danish; Ludvig Lorenz (1829-1891); internal validity; electric current; free electron model; free electrons; Debye temperature; Peter Debye (1884-1966); model for the heat capacity; X-ray diffraction; Nobel Prize in Chemistry; chemical element; Aluminum; Beryllium; Cadmium; Cesium; Diamond; Chromium; Copper; Germanium; Gold; Iron; Lead; Manganese; Nickel; Platinum; Rubidium; Sapphire; Selenium; Silicon; Silver; Tantalum; Tin (white); Titanium; Tungsten; Zinc; temperature dependence of the thermal conductivity of beryllium; celsius; °C; melting point; trend; electrical conductivity; chart; plot; Gnumeric; data; solid; liquids; open access paper; scientist; engineer; University of Virginia (Charlottesville, Virginia); European Commission; Joint Research Centre (Karlsruhe, Germany); Laser Thermal Analysis, Inc. (Charlottesville, Virginia); University of Rhode Island (Kingston, Rhode Island); differential; radiometry; technology; technique; tungsten; melting; molten; Physical Review Letters; research; chemical element; elemental; laser beam; center (geometry); millimeter; disk (mathematics); emissivity; emit; electromagnetic radiation; sensor; monitor; power (physics); area; calculation; Fourier's law; Schematic of the thermal conductivity apparatus and comparison data; schematic; experiment; experimental; scientific instrument; apparatus; crucible; scientific literature; Creative Commons Attribution 4.0 International license.