| Tikalon Blog is now in archive mode.
An easily printed and saved version of this article, and a link
to a directory of all articles, can be found below: |
|
This article |
| Directory of all articles |
Gel and Granular Flow
June 20, 2022
As a
Baby Boomer, I remember the t
television commercials that insisted that "There's always room for
Jell-O," the
trademark of a
popular gelatin dessert.[1] Aside from an occasional
beer on a
summer's night, I'm not much of a
drinker, so I've never had a
jello shot. The popular
Middle East confection,
Turkish delight is made from
corn starch, not
gelatin, but my
wife makes a version using
unflavored gelatin and
orange juice, and it's very good.
Recipes for gelatin-based Turkish delight can be found on the
Internet.
"Turkish Delight," xkcd comic 1980, by Randall Munroe (b. 1984). My children enjoyed The Chronicles of Narnia: The Lion, the Witch and the Wardrobe, the film adaptation of the C.S. Lewis novel, the The Lion, the Witch and the Wardrobe. (Licensed under a Creative Commons Attribution-NonCommercial 2.5 License. View on the xkcd website.)
While doing
research on
capacitance touch sensors, I made an
artificial finger from a
water gel of gelatin. A gelatin gel seemed to be a good
simulation of a finger both
mechanically and
electrically. The
human body is mostly
water,[2] as is a gelatin gel, which is usually mixed to a
ratio of 0.125
ounce to 1
cup of water. I wanted a
rigid finger, so I increased this to 0.25 ounce, which is the contents of a typical
consumer gelatin
packet. I used a finger of an
acetonitrile glove as a
casting mold.
Since I had this artificial finger and one of those
cardboard sleeves that
insulate your
hand from the
hot exterior of a thin
paper cup, I decided to try an
experiment on how well such sleeves protect your fingers from
heat. That's when I discovered that my water gel finger
melted at
temperatures not far above
room temperature, about 30-35
°C. Two
Japanese scientists from the
Tokyo University of Agriculture and Technology and
Tokyo Metropolitan University have recently
published a study demonstrating that melting gelatin, when heated from below, shows mechanical properties similar to falling
beds of
granular materials such as
sand.[3-4] Their study is published as an
open access paper in
Scientific Reports.[3]
Granular materials flow under
gravitational force while maintaining some
rigidity, as
landslides and
avalanches demonstrate, and their gravitational
instability is
unpredictable and not well understood.[3] The
complex properties of granular materials depend on the
friction between
grains, the size
dispersion of the grains, and the
shape of grains.[3] External
force propagate in localized
paths known as a
force chains, and it's been found that
jamming in granular systems is similar to the
glass transition.[3] Gels are like granular materials at
microscopic scale, since their
polymer or
protein chains are similar to the granular force chains that underpin the
solidity of granular materials.[4]
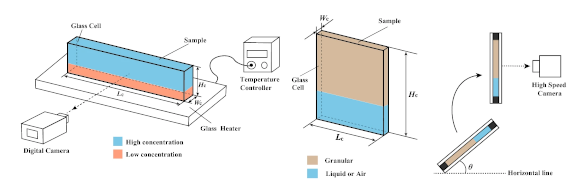
Left, the experimental setup for water gels. The glass sample chamber had dimensions of 30 mm x 126 mm x 2.4 mm. Right, the experimental setup for granular systems. There were two different sample chambers with dimensions 150 mm x 75 mm x 1.2 mm, and 90 mm x 65 mm x 2.4 mm with a manually set sedimentation angle. (Diagrams from ref. 3,[3] licensed under a Creative Commons Attribution 4.0 International License. Click for larger image.)
In their experiments on the gravitational instability of gels, the Japanese scientists used
high speed cameras to examine the
fluidization of thin beds of sand and gelatin
solutions.[4] In the sand experiments, beds of sand grains were formed in either
air or water, inverted, and then observed as the base began to fall out.[4] For gelatin, they prepared two layers of different
concentration, one on top of the other with the lower layer prepared to completely fluidize first.[4] When heated from below, the upper layer would eventually destabilize and fall.[4] The gelatin solutions were prepared with 3-14
wt% using pure water as a
solvent.[4] As an aid to
visualization,
tracer particles with a
density close to that of the gelatin were added at 0.05 wt%.[3]
images were recorded with a
digital camera at one
second intervals.[3]
In the experiments, a finger
pattern was observed that is much like the
Rayleigh–Taylor instability in fluids when a lighter fluid is pushing a heavier fluid.[3-4] In both the sand and gelatin systems, fingering instabilities were seen in which thin fingers of material fall into the lower material (or air/water), and these resemble
raindrops falling down a
window.[4] New fingers would appear in between existing ones over time, and the interface between the liquid and solid-like parts would recede.[4] One difference is that in granular materials, new fingers form between existing fingers, something that's not observed in fluid systems.[3]
The researchers found that falling sand and melting gelatin heated from below exhibit the same destabilization mechanism, and their destabilization
parameters scale with the
flowing, fluidized region thickness.[3-4] The thickness of this region depends on key parameters such as the
velocity of the receding
front, and the distance between the fingers, a relationship known as a
scaling law. Scaling laws connect
physical phenomena which seem completely different, but their
mechanisms are related at a deeper level.[4] In these materials, the existence of force-bearing networks connect their physical behavior.[4] It also appears that the
temporal evolution of the height of the fluidized layer is determined by the strength of the network.[3] This research will enhance our understanding of avalanches, landslides, and industrial flow processes, all of which can be destabilization under the force of gravity.[4]
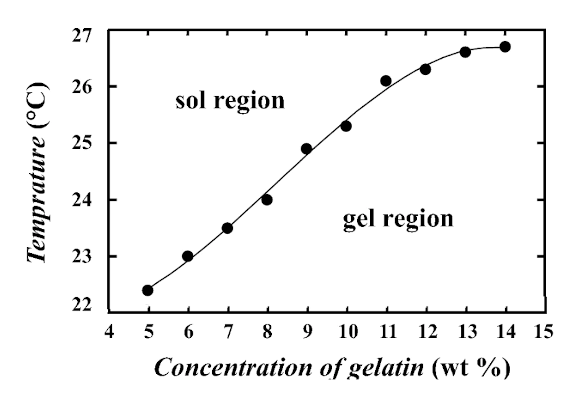
Dependence of the sol-gel transition temperature on gelatin concentration.
(Graph from ref. 3,[3] licensed under a Creative Commons Attribution 4.0 International License. Click for larger image.)
References:
- There's Always Room for Jell-O - Decades TV Network, YouTube Video by Retrospectacle, June 27, 2016.
- The Water in You: Water and the Human Body The United States Geological Survey, U.S. Department of the Interior.
- Kazuya U. Kobayashi and Rei Kurita, "Key connection between gravitational instability in physical gels and granular media," Scientific Reports, vol. 12, no. 6290 (April 15, 2022), https://doi.org/10.1038/s41598-022-10045-x. This is an open access article with a PDF file here.
- What do jelly and sand have in common?, Tokyo Metropolitan University Press Release, April 30, 2022.
Linked Keywords: Baby Boomer; television advertisement; television commercial; Jell-O; trademark; popular culture; popular; gelatin dessert; beer; summer; night; alcoholic drink; drinker; jello shot; Middle East; confection; Turkish delight; corn starch; gelatin; wife; flavor; unflavored; orange juice; recipe; Internet; xkcd comic; Randall Munroe (b. 1984); child; children; The Chronicles of Narnia: The Lion, the Witch and the Wardrobe; film adaptation; C. S. Lewis; novel; The Lion, the Witch and the Wardrobe; Creative Commons Attribution-NonCommercial 2.5 License; xkcd website; research; capacitance; touch switch; touch sensor; >artificial; finger; water gel (plain); simulation; mechanics; mechanically; electricity; electrical; human body; water; ratio; ounce; cup (unit); stiffness; rigid; consumer; packet (container); acetonitrile; molding (process); casting mold; cardboard; tube; sleeve; thermal insulation; insulate; hand; hot; paper cup; experiment; heat; melting; melted; temperature; room temperature; Celsius; °C; Japan; Japanese; scientist; Tokyo University of Agriculture and Technology; Tokyo Metropolitan University; scientific literature; publish; bed (geology); granular material; sand; open-access journal; open access paper; Scientific Reports; gravitation; gravitational force; rigidity; landslide; avalanche; instability; predictability; unpredictable; complexity; complex; material properties; friction; crystallite; grain; statistical dispersion; geometry; shape; force; propagation; propagate; Euclidean vector; path; jamming (physics); glass transition; microscopic scale; polymer; protein; polymer chain; solid; solidity; experimental setup; glass; sample (material); container; chamber; dimension; millimeter; mm; granular material; granular system; manual labor; manual; manually; sedimentation; angle; Creative Commons Attribution 4.0 International License; high speed camera; fluidization; aqueous solution; atmosphere of Earth; air; concentration; mass fraction (chemistry); wt%; solution; solvent; observation; visualization; flow tracer; particle; density; digital imaging; image; digital camera; second; pattern; Rayleigh–Taylor instability; rain; raindrop; window; parameters; fluid dynamics; flowing; velocity; front (physics); power law; scaling law; physics; physical; mechanism; time; temporal; gel transition temperature on gelatin concentration; dependent and independent variables; dependence; mechanics of gelation; sol-gel transition; There's Always Room for Jell-O.