Exfoliated Graphite
November 26, 2018
Lead, which was used in many
consumer applications, including as a
gasoline additive, has been banned since the middle of the
20th century because of its
toxicity. That's why many people are confused about the existence of
lead pencils. Lead pencils are actually made from
graphite, not lead. As explained in a informative
blog article,[1] the graphite
material of the first pencils was initially thought to be a form of lead, and it was called plumbago after the
Latin word for lead (plumbum, from which we get the
chemical symbol for lead, Pb).
Plumbago was discovered several
centuries ago as an
ore deposit in
Cumbria, England. It was extensively used as a
mold-release agent in the
casting of
cannon balls. While modern
analytical techniques were not available at that time, it should have been easy to distinguish graphite from lead by its
density. Graphite has a density of about 2.2
g/cc, while lead has a density of 11.34 g/cc. A large density difference would have been expected even if plumbago wasn't lead, but another
compound of lead, such as
lead carbonate, since lead carbonate's density is about 6.6 g/cc. Eventually, it was found that the material was not lead, but the term is still associated with pencils.

Detail of lead pencil construction. Modern pencil leads are formed as a composite of clay and graphite powder and are encased in wood. (Wikimedia Commons image, modified.)
Graphite makes an excellent pencil material, since flakes of
carbon are easily
rubbed from graphite because of its
layered structure. Graphite is composed of
sheets of
graphene loosely held atop each other by weak
van der Waals forces. The difference in
bond strength between layers can be seen by the difference in the carbon-carbon
atomic distance, 0.142
nm in the graphene
plane, and 0.335 nm between planes.
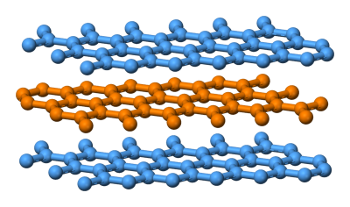
Layered structure of graphite.
Carbon atoms in the planes are tightly bonded, while the planes are bonded together by weak van der Waals forces.
(Wikimedia Commons image by Benjah-bmm27.)
The spacing between graphene layers is larger than the
size of many small
molecules.
water, for example, has a size of about 0.275 nanometers. For this reason, graphite can be
intercalated; that is, molecules and
ions can be inserted between the layers. Intercalation will slightly expand the gap between the graphene sheets.
Metal halides, such as
ferric chloride, FeCl
3, are common
compounds for graphite intercalation.
While intercalation does modify the
physical properties of graphite, taking intercalation to an extreme by causing a large layer separation leads to
exfoliation, a breakup of graphite into its individual layers. The term, exfoliation, comes from the word,
folio, which is an individual
sheet of paper. Exfoliation of graphite can be achieved by heating ferric chloride intercalated graphite at several hundred
degrees Celsius. One early application of exfoliated graphite was as a
high temperature gasket material (Grafoil®).[3] Since layers of graphite are the important electronic material, graphene, there has been much research in exfoliation of graphite.[4-6]
Exfoliation of graphite and layered compounds results in materials with
surface areas greater than 1000
square meters per
gram. Such materials will have enhanced
surface activity, and they are useful as
electrodes in
batteries and
supercapacitors. When used as part of a composite, exfoliated materials act as molecularly thin
barriers and
electromagnetic shields by virtue of their
conductivity.[4] Only a small quantity of
exfoliated graphene platelets, of the order of 2-5
weight percent, is required to make a shielding composite.
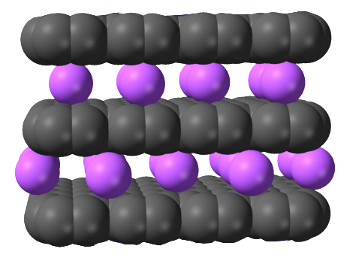
Graphite can be electrochemically loaded with potassium ions using an aqueous Potassium hydroxide (KOH) electrolyte and then exfoliated.[7]
In this illustration of graphite intercalated with potassium ions, the carbon atoms are naturally represented as black.
(Modified Wikimedia Commons image by Ben Mills.)
While graphene is seen as a breakthrough electronic material, it has a size problem.
Silicon wafers are as large as twelve
inches, and such sizes are needed to make
production of
integrated circuits economical, but graphene has only been produced in small flakes. That's why
research is being done on graphite exfoliation as one technique for production of large area graphene sheets. Exfoliation of graphene and similar materials is the topic of a recent
publication by researchers from the
Massachusetts Institute of Technology (Cambridge, Massachusetts),
MIT Lincoln Laboratory (Lexington, Massachusetts),
Yonsei University (Seoul, Republic of Korea),
Ohio State University (Columbus, Ohio), the
Georgia Institute of Technology (Atlanta, Georgia), the
University of Texas at Dallas (Richardson, Texas), the
University of Virginia (Charlottesville, Virginia), and the
University of Notre Dame (Notre Dame, Indiana).[8-9] Their exfoliation technique is different from
chemical techniques.
The
traditional way of removing graphene sheets from graphite is the one discovered by
Andre Geim and
Konstantin Novoselov, who were awarded the 2010
Nobel Prize in Physics for their pioneering work on graphene.[10] They used
cellophane tape to peel off single layers of graphene carbon from bulk graphite. As is easily realized, this method does not scale well to the production of large area graphene
substrates for
electronic devices.[8-9] It's also difficult to produce graphene layers on other materials since
nucleation is difficult.[8]
This new exfoliation process allows for the rapid
wafer-scale (5
centimeter diameter) production of not only
monolayers of carbon, but also monolayers of
hexagonal boron nitride (h-BN),
tungsten disulfide (WS2),
tungsten diselenide (WSe2),
molybdenum disulfide (MoS2), and
molybdenum diselenide (MoSe2).[8-9] First, a thick stack of monolayers one of one of these materials is
grown on top of a
sapphire wafer, then a 600-nanometer-thick
nickel film is
deposited onto the top of the stack.[9] Since the materials
adhere more strongly to the nickel than sapphire, lifting the nickel film removes the material stack.[9] When a second nickel film is deposited on the bottom of the stack, it's possible to peel off single-atom thick monolayers of those materials.[9] After this first monolayer is removed, the process can be repeated until the stack is depleted (see figure).[9]
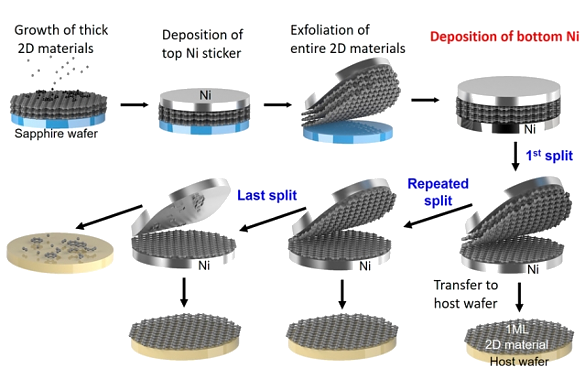
Graphene exfoliation process by which wafer-scale graphene is produced. (MIT image.)
The research team plans to demonstrate several electronic devices based on the monolayers produced by this process, including
flexible devices and a
nonvolatile memory array.[9] They've already fabricated working arrays of
field-effect transistors having just few atoms thickness.[9]
References:
- Stephanie Huesler, "Plumbago vs. Graphite," History Undusted, May 31, 2013.
- Minzhen Cai, Daniel Thorpe, Douglas H. Adamson and Hannes C. Schniepp, "Methods of graphite exfoliation," Journal of Materials Chemistry, vol. 22, no. 48 (September 12, 2012), pp. 24992-25002, DOI: 10.1039/C2JM34517J.
- James H Shane, Robert J Russell, and Raymond A Bochman, "Flexible graphite material of expanded particles compressed together," U.S. Patent No. 3,404,061, March 21, 1962.
- Valeria Nicolosi, Manish Chhowalla, Mercouri G. Kanatzidis, Michael S. Strano, and Jonathan N. Coleman, "Liquid Exfoliation of Layered Materials," Science, Vol. 340, no. 6139 (June 21, 2013), Article no. 1226419, DOI: 10.1126/science.1226419.
- D. D. L. Chung, "A review of exfoliated graphite," Journal of Materials Science, vol. 51, no. 1 (January, 2016), pp 554-568, https://doi.org/10.1007/s10853-015-9284-6.
- Richard E.Stevens, Sydney Ross, and Sheldon P.Wesson, "Exfoliated graphite from the intercalate with ferric chloride," Carbon, vol. 11, no. 5 (October, 1973), pp. 525-530, https://doi.org/10.1016/0008-6223(73)90312-6.
- Prashant Tripathi, Ch. Ravi Prakash Patel, M. A. Shaz, and O. N. Srivastava, "Synthesis of High-Quality Graphene through Electrochemical Exfoliation of Graphite in Alkaline Electrolyte," arXiv, October 28, 2013.
- Jaewoo Shim, Sang-Hoon Bae, Wei Kong, Doyoon Lee, Kuan Qiao, Daniel Nezich, Yong Ju Park, Ruike Zhao, Suresh Sundaram, Xin Li, Hanwool Yeon, Chanyeol Choi, Hyun Kum, Ruoyu Yue, Guanyu Zhou, Yunbo Ou, Kyusang Lee, Jagadeesh Moodera, Xuanhe Zhao, Jong-Hyun Ahn, Christopher Hinkle, Abdallah Ougazzaden, and Jeehwan Kim, "Controlled crack propagation for atomic precision handling of wafer-scale two-dimensional materials," Science (Early Release, October 11, 2018), Article no. eaat8126, DOI: 10.1126/science.aat8126.
- Helen Knight, "Researchers quickly harvest 2-D materials, bringing them closer to commercialization," MIT Press Release, October 11, 2018.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, "Electric Field Effect in Atomically Thin Carbon Films," Science, vol. 306, no. 5696 (October 22, 2004), pp. 666-669.
Linked Keywords: Lead; consumer; gasoline additive; 20th century; toxic; toxicity; pencil; lead pencil; graphite; blog; material; Latin; word; chemical symbol; century; ore deposit; Cumbria, England; mold-release agent; casting; round shot; cannon ball; analytical chemistry; density; kilogram per cubic meter; g/cc; chemical compound; lead carbonate; construction; composite; clay; powder; wood; Wikimedia Commons; carbon; rubbing; rub; layered structure; sheet; graphene; van der Waals force; bond energy; bond strength; bond length; atomic distance; nanometer; nm; lattice plane; Benjah-bmm27; molecular size; molecule; water; intercalation (chemistry); intercalate; ion; metal halide; iron(III) chloride; ferric chloride; physical property; folio; sheet of paper; degrees Celsius; high temperature; gasket; surface area; square meter; gram; surface energy; surface activity; electrode; battery; electric double-layer capacitor; supercapacitor; diffusion barrier; electromagnetic shielding; electromagnetic shield; electrical conductivity; exfoliated graphene platelet; weight; electrochemistry; electrochemical; potassium; qqueous solution; potassium hydroxide (KOH); electrolyte; silicon wafer; inches; manufacturing; production; integrated circuit; economics; economical; research; scientific literature; publication; Massachusetts Institute of Technology (Cambridge, Massachusetts); MIT Lincoln Laboratory (Lexington, Massachusetts); Yonsei University (Seoul, Republic of Korea); Ohio State University (Columbus, Ohio); Georgia Institute of Technology (Atlanta, Georgia); University of Texas at Dallas (Richardson, Texas); University of Virginia (Charlottesville, Virginia); University of Notre Dame (Notre Dame, Indiana); chemistry; chemical; tradition; Andre Geim; Konstantin Novoselov; Nobel Prize in Physics; cellophane tape; substrate; electronic device; nucleation; centimeter; diameter; monolayer; hexagonal boron nitride (h-BN); tungsten disulfide (WS2); tungsten diselenide (WSe2); molybdenum disulfide (MoS2); molybdenum diselenide (MoSe2); crystal growth; grow; sapphire; nickel; thin film; physical vapor deposition; deposit; adhesion; adhere; deflection; flexible; nonvolatile memory array; field-effect transistor.