Molecular Fountains
February 20, 2017
As a child in the
1950s, I got to see a lot of
movies in the back seat of my
parents'
car at the
drive-in theater. Our local drive-in was perhaps unique in having the rear of the giant outdoor
projection screen configured as a decorative
waterfall, lighted with
colored lights as was
Niagara Falls.[1] One film I attended, but likely didn't understand, was the 1954
Three Coins in the Fountain, known also for the
Frank Sinatra song of the same name.[2]

The fountain of the movie, Three Coins in the Fountain, is Trevi Fountain, located in Rome, Italy. About three thousand dollars per day of coins are thrown into the fountain, and the coins are harvested to support a food bank for the poor. (Wikimedia Commons photo by Andrew Chen.)
Fountains figure mightily in the
Biblical account of
Noah's flood, in which "...all the fountains of the great deep burst forth..." (
Genesis 7:11). The
Cassini spacecraft, in a 2005
flyby of
Saturn's moon,
Enceladus, discovered huge (up to 200
kilometer)
fountains of
water vapor and
ice particles.
Closer to home, the
ammonia fountain is a simple
chemistry experiment that demonstrates the high
solubility of
ammonia gas in
water. Introduction of a small quantity of water into an ammonia-filled
flask causes formation of a
partial vacuum that draws more water into the flask from a lower
reservoir, and that causes an increased
suction of more water to dramatic effect.[3]
An impressive fountain arises from the introduction of
Mentos candy into a
bottle of
Diet Coke to cause a
Diet Coke and Mentos eruption. This fountain is interesting, since the mechanism for
bubble formation is
physical, and not
chemical. The Mentos introduces
nucleation sites for bubbles of
carbon dioxide gas that exists in the
carbonated beverage.
While the ammonia and Diet Coke fountains illustrate chemical and physical laws, they're not that useful
technologically. There is one fountain, the
atomic fountain, that forms the basis of extremely accurate
clocks. If we force
atoms to flow upwards in a fountain, they will eventually fall downwards under the influence of
gravity. As the atoms fall, they are
weightless, and this reduces the uncertainty in a particular
measurement.
The
Ramsey method was
invented by
physicist,
Norman Foster Ramsey Jr. (1915-2011), who was awarded the 1989
Nobel Prize in Physics for this
research. This method is a technique to
synchronize an
oscillator to an
electron transition in a cloud of atoms. Application of this method is complex, but it allows an accurate measurement of the percentage of atoms that were induced to transition at an applied
microwave frequency. In this way, the difference between the applied frequency and the transition frequency can be determined, and the applied frequency can be adjusted to equal the transition frequency.
Accuracy in the frequency measurement is enhanced when the atoms in the cloud are motionless. At the peak of a fountain, the movement of atoms is small, so an atomic fountain allows creation of a highly accurate
atomic clock. One such clock is the
NIST-F1 at the US National Bureau of Standards and Technology (see photo). This clock, active since 1999, measures a
hyperfine transition in a fountain of
cesium-133 atoms. The
second is defined as 9,192,631,770 periods of this transition.
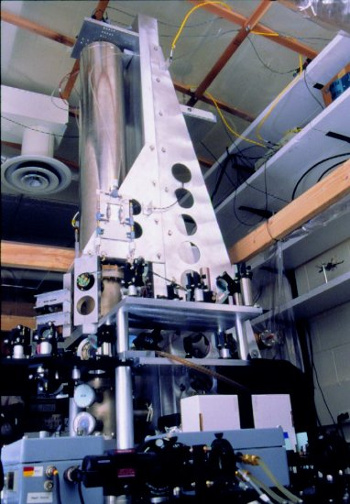
The NIST-F1 cesium atomic clock in 1999. A companion to NIST-F1, NIST-F2, was activated in April, 2013.
While there are still some problems that need correction, NIST-F2 has a goal of about a second's error in 300 million years.
(National Institute of Standards and Technology, Physics Laboratory, Time and Frequency Division, photograph, via Wikimedia Commons.)
While generating atomic fountains may seem complicated, an atomic fountain is far easier to create than a fountain of
molecules. First, cesium and its companion
frequency standard element,
rubidium, are
singly-charged species. Second, as
spherical atoms, there's no complication from
rotational forces. The impetus for creation of a molecular fountain is that
spectrographic and
interferometric resolution in
experiments is ultimately limited by how long a particle can be examined. Putting molecules in a fountain allows for long observations and better resolution.[4-5] Of what use is such enhanced resolution? It can yield highly accurate measurements of the
proton-to-electron mass ratio; and, perhaps, reveal a permanent
electric dipole moment of the electron.[6]
The first molecular fountain, of
ammonia molecules, was created by physicists at
Vrije Universiteit (Amsterdam, The Netherlands).[4-6] While atomic fountains reduce the
velocity of their atoms by
laser cooling, this technique won't work for molecules because of their internal
energy structure.[6] In the molecular fountain, the molecules are cooled using time-dependent
inhomogeneous electric fields applied by
electrodes.[6] As shown in the figure, an electrode structure called a
Stark decelerator removes energy from the molecular beam to slow it down.[6]
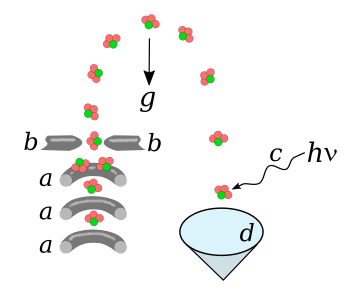
A molecular fountain of ammonia molecules.
Gravitational acceleration is indicated as g.
(a) Stark decelerator.
(b) Positioning electrodes.
(c) ionizing laser beam.
(d) Microchannel plate detector.
(Drawn using Inkscape.)
In the molecular fountain experiments, the applied electric fields were able to decelerate the molecules to speeds between 1.4 and 1.9
meters per second. After they peaked in the fountain, they were observed as they fall back under gravity.[4-5] The corresponding transverse
temperature of the molecules at that time was less than, or equal to, 10 μK, and the longitudinal temperature was less than, or equal to, 1 μK.[4-5]
The molecular free-fall period was up to 266
milliseconds, which allowed
sub-hertz frequency mesurements.[4-5] This measurement time is more than a hundred times larger than that provided by other techniques.[6] The molecules were
ionized using a
laser beam and detected to differentiate them from molecules that are present in the background gas.[6] At this time, just a single molecule can be examined every five fountain launches, so less than a molecule per second can be examined.[6] Future improvements are expected.[6]
References:
- You can also tell my age by my use of "lighted," and not "lit," as the past tense of light, an explanation of which can be found at the Grammar Girl Web Site.
- Three Coins in the Fountain (1954, Jean Negulesco, Director) on the Internet Movie Database.
- Scott Milam, "Ammonia Fountain Demo," YouTube Video, January 27, 2015.
- Cunfeng Cheng, Aernout P. P. van der Poel, Paul Jansen, Marina Quintero-Pérez, Thomas E. Wall, Wim Ubachs, and Hendrick L. Bethlem, "Molecular Fountain," Phys. Rev. Lett., vol. 117, no. 25 (December 13, 2016), document 253201, DOI:https://doi.org/10.1103/PhysRevLett.117.253201.
- Cunfeng Cheng, Aernout P. P. van der Poel, Paul Jansen, Marina Quintero-Pérez, Thomas E. Wall, Wim Ubachs, and Hendrick L. Bethlem, "Molecular Fountain," arXiv, November 11, 2016.
- Noah J. Fitch, "Viewpoint: What Goes Up Must Come Down," Physics, vol. 156 (January 9, 2017).
Permanent Link to this article
Linked Keywords: 1950s; film; movie; parent; automobile; car; drive-in theater; projection screen; waterfall; colored; electric light; Niagara Falls; Three Coins in the Fountain; Frank Sinatra; Three Coins in the Fountain (song); fountain; Trevi Fountain; Rome, Italy; dollar; coin; food bank; poverty; poor; Wikimedia Commons; Bible; Biblical; Genesis flood narrative; Noah's flood; Genesis 7:11; Cassini spacecraft; Enceladus flyby; Moons of Saturn; Enceladus; kilometer; south polar plume; water vapor; ice; ammonia fountain; chemistry; experiment; solubility; ammonia gas; water; Erlenmeyer flask; partial vacuum; reservoir; suction; Mentos candy; bottle; Diet Coke; Diet Coke and Mentos eruption; bubble; physics; physical; chemistry; chemical; nucleation site; carbon dioxide gas; carbonation; carbonated; beverage; technology; technologically; atomic fountain; clock; atom; gravitation; gravity; weightlessness; weightless; measurement; Ramsey interferometry; Ramsey method; invention; invented; physicist; Norman Foster Ramsey Jr. (1915-2011); Nobel Prize in Physics; research; synchronization; synchronize; electronic oscillator; atomic electron transition; microwave frequency; accuracy; atomic clock; NIST-F1; hyperfine structure; hyperfine transition; cesium-133; second; cesium; NIST-F2; measurement uncertainty; error; molecule; frequency standard; chemical element; rubidium; electric charge; singly-charged; sphere; spherical; rotation; rotational; moment of inertia; force; spectrograph; spectrographic; interferometry; interferometric; spectral resolution; proton-to-electron mass ratio; electric dipole moment of the electron; Vrije Universiteit (Amsterdam, The Netherlands); velocity; laser cooling; energy; inhomogeneous; electric field; electrode; molecular beam; Stark decelerator; ionization; ionizing; laser beam; microchannel plate detector; Inkscape; meters per second; temperature; millisecond; hertz; sub-hertz.