Cold Work on the Nanoscale
January 2, 2017
My first
metallurgy lesson came early in life as I was bending
aluminum to make
chassis for
electronic circuitry. I started building electronic circuits in
elementary school using components harvested from old
television receivers. The receivers of that period, and my circuits, were built from
vacuum tubes. I could build an
audio amplifier from just four or five vacuum tubes.
Soldering wires was the easy part. The hard part was
machining holes in an
aluminum chassis to mount the tube
sockets,
transformers, and
controls.
Transformers typically required a large
square hole, and my only
tool for making these was an
electric drill. I would
drill a chain of small holes near the
perimeter of the square, and cut a path through the line of holes using a small
file, a tedious task. Eventually, I discovered that I only needed to cut three sides of the square. Repeatedly
bending the aluminum back and forth would easily
break off the final side. I had discovered
work hardening.
As everyone familiar with
cooking foil and
beverage cans knows, aluminum is a
soft metal. It's easily created in its pure
elemental form by
electrolytic reduction of
aluminum oxide dissolved in a
molten solution by a
modified Hall process.[1] Pure metals, as
cast and distinguished from
alloys, are soft. The repeated bending (cold-working) forms
dislocations in the
crystal lattice that change the aluminum from a
ductile to a
hard and
brittle material.
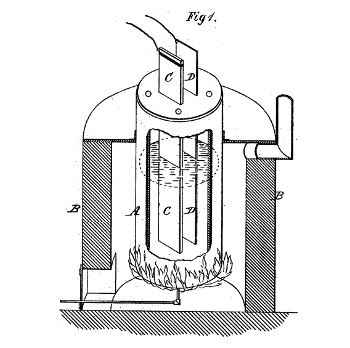
The inventive part of the electrolytic extraction of aluminum was finding a way to put it into a solution. Charles Hall discovered that alumina would dissolve in molten fluorides.
(Fig. 1 of US Patent No. 400,664, "Process of reducing aluminium from its fluoride salts by electrolysis," by Charles M. Hall, April 2, 1889.)
Forging and
shot peening are two of the more useful applications of work hardening. In forging, a component of near-net shape is
hammered into a
die to bring it to its desired shape. The forging process hardens the material by cold working, but forging is often done
at higher temperature to keep the material
ductile. A
video of cold forging of aluminum can be found in ref. 2.[2]
Peening is the process of hammering the
surface of a metal to increase the surface hardness. The process of hammer peening was used by
blacksmiths for
centuries without an understanding of why it works. Some readers may have seen a
ball peen hammer, but wondered about its purpose (see photograph).
Shot peening is a more rapid process that pelts a surface with a stream of hard
beads to provide the work hardening.

Ball peen hammer.
My maternal grandfather, who was a machinist, had one of these, and I wondered why it had such a strange shape.
(Via Wikimedia Commons.)
Surprisingly, shot peening was only invented about a
century ago, probably because there wasn't enough demand for a rapid peening process before
mass production of such items as
automobiles. Shot peening not only hardens the surface, but it puts the surface in
compression, and this
compressive force prevents surface
cracks from growing, so it will increase
fatigue life. Shot peening is mostly used in the production of
gears,
crankshafts and their associated
cams, and
turbine blades.
The
shot used is peening is chosen to be much harder than the peened material, but what about the
complementary system of soft shot against a hard target? That's the approach taken by
materials scientists and
mechanical engineers at
Rice University (Houston, Texas) and the
University of Massachusetts, Amherst, as a way to modify the
mechanical properties of
silver nanoparticles.[3-5] The results of their
research have been published as a recent article in
Science.[3] The research was led by Rice materials scientist,
Edwin Thomas, and the lead
author of the
paper is
Ramathasan Thevamaran, a Rice
postdoc.[4]
The silver particles used for these
experiments needed to be as perfect as possible, so they were
synthesized as
single crystals by
bottom seeded crystal growth.[4] The resulting
nanoscale cubes were about 1.4
micrometers on a side, and they were shot at a hard
silica target at about 400
meters per second using a
laser technique.[3] This laser-induced projectile impact test (LIPIT) was developed at Rice University in 2012 for experiments on
polymer and
graphene film materials.[5-6]
The LIPIT system works by rapid laser heating of a thin
gold film beneath a polymer film on which the cubes are placed. The laser
power vaporizes the gold film, and the resulting expansion of the polymer film launches the nanocubes.[4] The laser apparatus was designed to accurately deposit the cubes at a desired
orientation so the affect of impacts at every
angle could be determined.[4] The silver cubes hit the silica surface at
supersonic velocity, transforming their
momentum into a huge
deformation energy over a half
millimeter travel distance.
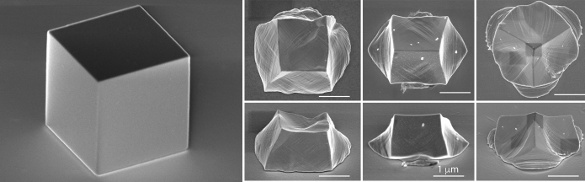
Single crystal silver cubes, such as the one on the left, are deformed on impact with a hard silica surface (right). (Still images from a YouTube Video by Rice University.)[7)]
The cubes were initially at
room temperature, but impact increased the temperature by about 350 degrees
Fahrenheit, a temperature which allowed dynamic
recrystallization of the silver.[4] Says Thomas,
"The high-velocity impact generates very high pressure that far exceeds the material's strength... This leads to high plasticity at the impact side of the cube while the top region retains its initial structure, ultimately creating a grain-size gradient along its height."[4]
The resulting high
strain rates, strain gradients, and recrystallization from these impacts created a gradient nano-grained structure from the nearly defect-free single-crystal nanocubes.[3-4] The gradient was at least an
order of magnitude greater than what has been produced by other techniques.[4]
Electron microscope analysis of the cubes eight days after impact showed that the gradient structure was still extant.[4]
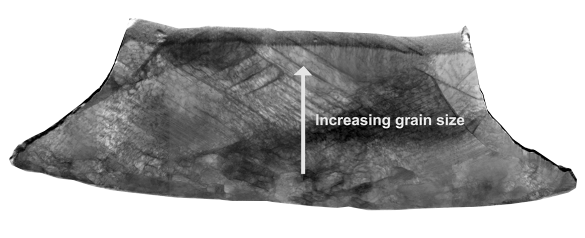
Image showing grain boundary lines in the strain gradient of a silver nanocube. (Still image from a YouTube Video by Rice University.[7]
Strain gradients such as those produced can create ductile and
tough metals.[3] Such a processing technique might give materials with high strength that are less susceptible to
brittle fracture.[4] The technique might be used to enhance
cold spray processing.[4]
References:
- Charles M. Hall, "Process of reducing aluminium from its fluoride salts by electrolysis," US Patent No. 400,664, April 2, 1889.
- Aluminium Forging, YouTube Video, June 29, 2011.
- Ramathasan Thevamaran, Olawale Lawal, Sadegh Yazdi, Seog-Jin Jeon, Jae-Hwang Lee, and Edwin L. Thomas, "Dynamic creation and evolution of gradient nanostructure in single-crystal metallic microcubes,"Science, vol. 354, no. 6310 (Oct 21, 2016), pp. 312-316, DOI: 10.1126/science.aag1768
- Mike Williams, "Smashing metallic cubes toughens them up," Rice University Press Release, October 20, 2016.
- Jae-Hwang Lee, David Veysset, Jonathan P. Singer, Markus Retsch, Gagan Saini, Thomas Pezeril, Keith A. Nelson, and Edwin L. Thomas, "High strain rate deformation of layered nanocomposites," Nature Communications vol. 3 (November 6, 2012), Article no. 1164, doi:10.1038/ncomms2166.
- Mike Williams, "Microbullets reveal material strengths, Rice University Press Release, October 30, 2012.
- Smashing silver micro-cubes toughens them up, Rice University YouTube Video, October 20, 2016.
Permanent Link to this article
Linked Keywords: Metallurgy; aluminum; chassis; electronic circuitry; elementary school; television set; television receiver; vacuum tube; audio amplifier; solder; soldering; wire; machining; aluminum; electrical connector; socket; transformer; potentiometer; control; square; tool; electric drill; drilling; perimeter; file; bending; fracture; break; work hardening; aluminium foil; cooking foil; beverage can; ductility; metal; chemical element; elemental; electrolytic cell; electrolytic reduction; aluminum oxide; solution; dissolve; melting; molten; modified Hall process; casting; cast; alloy; dislocation; crystal structure; crystal lattice; hardness; hard; brittleness; brittle; material; invention; inventive; electrolytic extraction; Charles Martin Hall; alumina; fluoride; US Patent No. 400,664; Forging; shot peening; hammer; hammered; die; hot working; video clip; surface; blacksmith; century; centuries; ball peen hammer; bead; grandparent; maternal grandfather; machinist; Wikimedia Commons; mass production; automobile; compression; compressive force; crack; fatigue life; gear; crankshaft; cam; turbine blade; shot; complementary; materials science; materials scientist; mechanical engineering; mechanical engineer; Rice University (Houston, Texas); University of Massachusetts, Amherst; mechanical properties; silver; nanoparticle; research; Science; Edwin Thomas; author; scientific literature; paper; Ramathasan Thevamaran; postdoctoral research; postdoc; experiment; chemical synthesis; synthesize; single crystal; seed crystal; bottom seeded; crystal growth; nanoscopic scale; nanoscale; cube; micrometer; silicon dioxide; silica; meters per second; laser; polymer; graphene; gold; power; vaporization; vaporize; orientation; angle; Mach number; supersonic velocity; momentum; deformation; energy; millimeter; single crystal; YouTube Video; room temperature; Fahrenheit; recrystallization; pressure; strength; plasticity; grain-size; gradient; strain rate; order of magnitude; electron microscope; analysis; grain boundary; strain; toughness; tough; brittle fracture; gas dynamic cold spray; cold spray processing; Charles M. Hall, "Process of reducing aluminium from its fluoride salts by electrolysis," US Patent No. 400,664, April 2, 1889.