Humidity Sensing
June 13, 2016
In
elementary school, one of the first
experiments I performed with my home
chemistry set was creation of a
humidity indicator.
Students of
my generation were able to experiment with a range of
chemical compounds that are not sold to today's
children for
safety reasons, and one such compound was the
cobalt chloride that enabled this humidity
sensor. Cobalt chloride is a suspected
carcinogen. While it seems as if everything tested is found to cause
cancer, this chemical is also a common
allergen, so I agree that it's best to keep this chemical away from children.

A 1940s era Gilbert chemistry set.
Cobalt chloride was likely included in this set, as well as other experimental staples as sodium ferrocyanide and phenolphthalein.
(Wikimedia Commons image by Jmabel.)
My chemical humidity sensor was just a blot of cobalt chloride solution on
paper. Its principle was the difference in
color between the
hydrated form of cobalt chloride, cobalt chloride hexahydrate, CoCl
2·6H
2O, and the
anhydrous form without the
waters of hydration. The hexahydrate has a deep
purple color, while the anhydrous form is
sky blue. The water in the air was in
equilibrium with the water in the hydrate, so the chemical blot was blue when the air was dry, and generally
pink when the air was wet. As I remember, I wasn't really impressed by the shallow change in color that I saw.
Humidity indicators using cobalt chloride were made
commercially from the late
1940s onwards, principally as a
quality control measure for
military supplies. One such humidity indicator was
patented in 1952.[1] In that indicator, a
porous silica gel was impregnated with about 2-5% anhydrous cobalt chloride by
dry weight. This gel was then dried for placement in shipping containers. Less
hazardous humidity indicators are based on
copper chloride, CuCl
2, which is
yellow in its anhydrous state, and
blue in its di-hydrate form (CuCl
2·2H
2O).

Anhydrous (left) and hydrated (right) cobalt(II) chloride.
(Left image, and right image, by Wilco Oelen, via Wikimedia Commons.)
There are quite a few other techniques, aside from the color change of a chemical, to make a
hygrometer; that is, a humidity
gauge. The simplest sort was invented by
Leonardo da Vinci in 1480. His hygrometer was a
pan balance with a wad of
cotton on one pan, and a
counterweight on the other. As the cotton absorbs water from the
atmosphere, its weight increases, so the humidity can be measured by a change in weight.
Another
mechanical method uses the change in
length of
fibers, such as
horsehair and
human hair, as they absorb water from the air. Since the change in length is small, a better technique is to observe the
twist angle of a twisted fiber such as
wool thread. In an action similar to that of a
bimetallic thermometer, a
salt-impregnated paper glued to a coil of
metal or another
elastic material that doesn't absorb water will enable a low cost and reasonably
accurate gauge device for humidity.
Rapunzel might have donated a strand of her hair to make this hygrometer, but it's more likely that cotton twine was used.
The mechanism is easy to understand.
(A 1688 copper engraving on paper by Joachim d' Alencé, Accession number df_tg_0004700 of the Deutsche Fotothek, via Wikimedia Commons.)
Modern
electronics has facilitated many precise methods of humidity measurement, such as
chilled-mirror dew point hygrometers. As everyone who has needed to defog an
automobile windshield on a cold
summer's morning has noted, lowering the
temperature will
condense moisture from the air. If we cool a surface, the temperature at which water starts to condense is called the
dew point. As shown in the following graph, the dew point will give the
relative humidity at a particular air temperature.
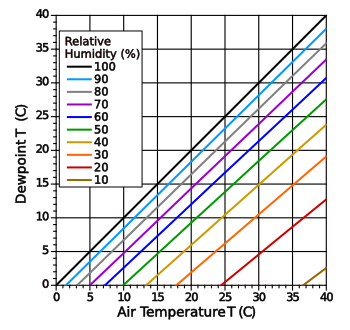
Relative humidity and dew point relationship.
The curves are calculated using the Magnus-Tetens approximation.
(Modified Wikimedia Commons image by Easchiff.)
Electronic chilled mirror dew point hygrometers function by monitoring the
reflection of
light from a
mirror as it's chilled. These hygrometers are easy to build using available electronic components, as shown in the figure. Unfortunately,
pollutants in the air will build up on the mirror after repeated cycling through the dew point, so this type of hygrometer requires continued
maintenance.
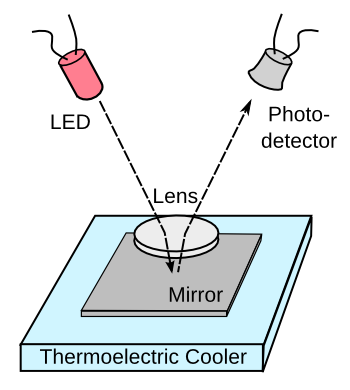
A chilled-mirror dew point hygrometer.
The components shown are quite inexpensive, although the thermoelectric cooler requires a fanned heat sink to extract heat from the Peltier hot junctions, and some microcontroller circuitry is needed for temperature control, data acquisition, and data analysis.
(Created using Inkscape.)
Some
material properties are sensitive to humidity. For example, the
dielectric constant of some
polymers and metal
oxides will change with humidity, enabling
capacitive hygrometers. The
electrical conductivity of some salts and
conductive polymers changes with humidity, but it changes also with temperature. The
thermal conductivity of air increases with humidity; but, as can be imagined, hygrometers based on thermal conductivity are difficult to build.
While we congratulate ourselves on the various ways we've devised to measure humidity, the lowly
fruit fly (Drosophila melanogaster) also measures humidity. A team of
scientists from
Northwestern University (Evanston, Illinois),
Lund University in
Sweden, and the
New York University School of Medicine, have found that fruit flies, which prefer the distinct humidity range of their native
habitat, sense relative humidity through
neurons in an small
organ structure, a sac in their
antennae known as the sacculus. Relative humidity and temperature are processed by different cells in the Drosophila antenna.[2-3]
The
research team investigated the
genes and neurons necessary for hygrosensation in the common fruit fly, Drosophila melanogaster. It's quite
logical, from an
evolutionary standpoint, that
insects should possess a "sixth sense" for detecting water vapor in the air, since such a sense would direct them to the most favorable
environment.[2] Says
Marcus C. Stensmyr, an
associate professor at Lund University and a
co-author of the study,
"That insects are able to detect humidity levels has been known since the beginning of the 20th century, but how they do it has remained enigmatic... Our study reveals for the first time the genes and neurons that underlie this ability, which is very exciting."[3]
It appears that fruit flies sense humidity by the same principle as hygrometers utilizing
tension on strands of hair. The mechanical
deformation of the sacculus is likely the means of fruit fly humidity sensing.[3] This study may help in the design of strategies for
mosquito population control, such as a means to prevent the insects from finding water in which to lay their
eggs.[3]
Says
Marco Gallio, an assistant professor of
neurobiology at Northwestern University and a co-author of the study, "Our discovery is very important for
sensory biology and offers a possible tool for fighting mosquitoes and the
disease they can carry."[3] This work received funding from the
National Institutes of Health.[3]
References:
- Paul Bell Davis, "Cobalt chloride humidity indicator," US Patent No. 2,580,737, January 1, 1952 (via Google Patents).
- Anders Enjin, Emanuela E. Zaharieva, Dominic D. Frank, Suzan Mansourian, Greg S.B. Suh, Marco Gallio, and Marcus C. Stensmyr, "Humidity Sensing in Drosophila," Cell (In Press, May 5, 2016), DOI: http://dx.doi.org/10.1016/j.cub.2016.03.049.
- Megan Fellman, "Scientists Are First to Discover Sensory System That Detects Air Humidity," Northwestern University Press Release, May 5, 2016.
Permanent Link to this article
Linked Keywords: Elementary school; experiment; chemistry set; humidity; indicator; student; baby boomer; my generation; chemical compound; child; children; safety; cobalt chloride; sensor; carcinogen; cancer; allergen; sodium ferrocyanide; phenolphthalein; Wikimedia Commons; Jmabel; paper; color; hydrate; hydrated; anhydrous; water of crystallization; waters of hydration; purple; sky blue; chemical equilibrium; pink; commerce; commercial; 1940s; quality control; military; patent; patented; porosity; porous; silica gel; dry weight; hazardous; copper chloride; yellow; blue; anhydrous; Wilco Oelen; hygrometer; gauge; Leonardo da Vinci; pan balance; cotton; counterweight; atmosphere; mechanics; mechanical; length; fiber; horsehair; human hair; twist; angle; wool; thread; bimetallic strip; thermometer; acid salt; metal; elasticity; elastic; material; accuracy; accurate; Rapunzel; twine; copper engraving; Deutsche Fotothek; electronics; chilled-mirror; dew point; automobile windshield; summer; morning; temperature; condensation; condense; relative humidity; curve; calculation; calculated; Magnus-Tetens approximation; Easchiff; reflection; light; mirror; air pollution; pollutant; maintenance; thermoelectric cooler; fanned heat sink; heat; Peltier; junction; microcontroller; electronic circuit; circuitry; temperature control; data acquisition; data analysis; Inkscape; material properties; relative permittivity; dielectric constant; polymer; oxide; capacitance; capacitive; electrical conductivity; conductive polymer; thermal conductivity; fruit fly (Drosophila melanogaster); scientist; Northwestern University (Evanston, Illinois); Lund University; Sweden; New York University School of Medicine; habitat; neuron; organ; antenna; research; gene; logic; logical; evolution; evolutionary; insect; environment; Marcus C. Stensmyr; associate professor; author; 20th century; enigma; enigmatic; tension; deformation; mosquito; population control; egg; Marco Gallio; neurobiology; sensory ecology; sensory biology; disease; National Institutes of Health; Paul Bell Davis, "Cobalt chloride humidity indicator," US Patent No. 2,580,737, January 1, 1952.