Electroluminescence
December 14, 2012
Robert Duvall, who is best known to
science fiction fans for his role in
THX 1138 (1971,
George Lucas, Director), had a somewhat earlier science fiction role. In
The Invaders, episode 20, season 1, of
Voyage to the Bottom of the Sea (1965,
Sobey Martin, Director;
Irwin Allen, Creator), he played Zar, a survivor from the
Earth of millions of years ago.
Earthmen of that time were quite superior to us, since Zar is able to learn
English in just a few hours. Before Zar shows his sinister side, he's taken on a tour of the
Seaview, the futuristic
research submarine that discovered him in
suspended animation. Noticing the ship's
incandescent lighting, Zar comments how the present-day Earthmen had not yet learned how to create
light without
heat.
Even in those early days, before widespread manufacture of
light-emitting diodes, there was a decade's old
solid state technology available for production of light without a heated
filament. This was
electroluminescence, which was discovered in
silicon carbide in 1907, and in
copper-
doped zinc sulfide (ZnS:Cu) in 1923.[1] By the 1960s, to
paraphrase Henry Ford, you could have any inefficient solid state light you wanted, as long as it was
red (LED), or
green (electroluminescent). We needed to wait until the 1990s for the first
white LEDs.
Despite its solid state advantage, electroluminescence wasn't used that much as a light source because it was
inefficient, as the table shows. Electroluminescent devices also require a high
voltage supply to operate. Nowadays, local generation of high voltages is not a problem with our miniature
switching power supplies, but it was a problem in the sixties. Nevertheless, electroluminescent panels were a popular
backlighting option, and they functioned well for some signage. Alas, they were not intense enough for room lighting, even if white light had been an option.
As in all electrically-driven light sources, electroluminescence arises from the recombination of
electrons and
holes, producing
photons. These
electron-hole pairs are produced by the intense electric field placed across the material. Unlike diode light sources, the voltage supplied to electroluminescent devices is of the
alternating current type, typically about a thousand
volts per
millimeter.
This is quite a high voltage, since the
dielectric strength of most
inorganic materials is of the order of 10,000 volts per millimeter.
Mica is an exception, since its strength is more than a 100,000 volts per millimeter, and that's why it's useful in many
radio frequency circuits.
It's possible to make a white electroluminescent light through the use of red, green and
blue materials. Green can be produced from copper-doped zinc sulfide, as mentioned above; zinc sulfide doped with
manganese (ZnS:Mn) can produce an orange-red color in thin film form; and zinc sulfide doped with
silver (ZnS:Ag) will produce blue. All of these are low efficiency materials.
A team of
physicists from the
Center for Nanotechnology and Molecular Materials,
Wake Forest University (Winston-Salem, NC) and
Trinity College (Dublin, Ireland) have developed an electroluminescent light with more than twice the efficiency of
compact fluorescent lamps, and about the same efficiency as LEDs.[2-4] These devices, which were under development for about two years, are so easy in their manufacture that there are plans for a first production run in 2013.[2,4]

Wake Forest University physics professor David Carroll (right), and Greg Smith, working on their new field-induced polymer electroluminescence lighting technology.
(Wake Forest University photograph by Ken Bennett, used with permission))
The device, based on a
field-induced polymer electroluminescence, is formed from
nanoparticles in a polymer matrix.[2,4] The emissive layer has
multi-walled carbon nanotubes (MWCNTs) dispersed in poly-(
N-vinylcarbazole) doped with
fac-tris(2-
phenylpyridine)
iridium(III). The MWCNTs are present at 0.04 weight-%. The polymer without the nanotubes is also electro-emissive, but at a fifth the emissivity.[3] The device structure is shown in the figure.
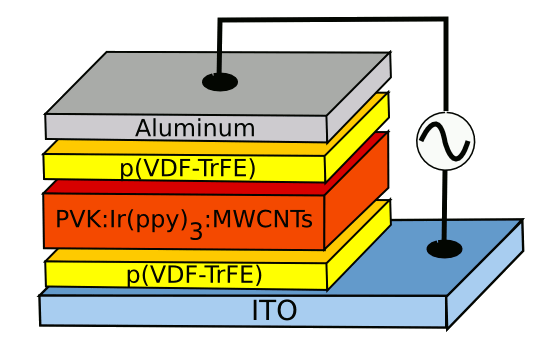
Wake Forest University electroluminescent device. ITO is the transparent indium-tin-oxide electrode, and PVF-trFE is polyvinylidene fluoride-trifluoroethylene. The emissive layer is described in the text. (Image by the author, rendered with Inkscape.)
This device is easily made into a large are emitter, and similar devices have a achieved a lifetime of at least a decade.[2,4]
David Carroll, leader of the research group, thinks that this device will supersede
organic light-emitting diodes for room lighting application.[4]
The only practical problem I see is the use of
indium, a rare and expensive metal, for the transparent electrode. When we're considering many square feet for a lighting unit, this would be a problem. As I discussed in a
previous article (Transparent and Conductive, June 10, 2011), research is ongoing for alternative transparent conductors.
References:
- Jeffrey A. Hart, Stefanie Ann Lenway and Thomas Murtha, "A History of Electroluminescent Displays," Indiana University Web Site, September 1999.
- Katie Neal, "Taking the buzz out of office lights," Wake Forest University Press Release, December 3, 2012.
- Yonghua Chen, Gregory M. Smith, Eamon Loughman, Yuan Li, Wanyi Nie and David L. Carroll, "Effect of multi-walled carbon nanotubes on electron injection and charge generation in AC field-induced polymer electroluminescence," Organic Electronics, vol. 14, no. 1 (January, 2013), pp. 8-18.
- Matt McGrath, "Plastic bulb development promises better quality light," BBC News, December 3, 2012.
Permanent Link to this article
Linked Keywords: Robert Duvall; science fiction; THX 1138; George Lucas; The Invaders; Voyage to the Bottom of the Sea; Sobey Martin; Irwin Allen; Earth; English; Seaview; research; submarine; suspended animation; incandescent lighting; light; heat; light-emitting diode; solid state technology; filament; electroluminescence; silicon carbide; copper; doping; doped; zinc sulfide; paraphrase; Henry Ford; red; green; white LED; luminous efficacy; voltage; switching power supply; backlighting; Lumens/Watt; efficiency; combustion; candle; electroluminescent; incandescent; fluorescent; light-emitting diode; LED; electro; hole; photon; electron-hole pair; alternating current; volt; millimeter; dielectric strength; inorganic compound; inorganic material; mica; radio frequency; circuit; blue; manganese; silver; physicist; Center for Nanotechnology and Molecular Materials; Wake Forest University (Winston-Salem, NC); Trinity College (Dublin, Ireland); compact fluorescent lamp; David Carroll; Ken Bennett; electric field; polymer; nanoparticle; multi-walled carbon nanotube; N-vinylcarbazole; phenyl; pyridine; iridium; indium-tin-oxide; polyvinylidene fluoride; trifluoroethylene; Inkscape; OLED; organic light-emitting diode; indium.