Imaging Atoms
September 26, 2012
If there was anyone who was still skeptical of the
atomic theory of matter at the end of the
twentieth century, they should have been convinced by the mass of
photographic evidence that started to appear at that time. Sure,
Xray diffraction suggests that matter is made from
atoms arrayed in space, but that's not an image of an actual atom. At the end of the century, scientists were able to image atoms, themselves.
Atoms have now been imaged for quite a few decades. Atoms of the
heavy elements, such as
tungsten and
uranium, were the first to be imaged using electron microscopy. These were somewhat easy targets, since they're
relatively large and packed full of
electrons. The
covalent radius of tungsten atoms is about 162
picometers (pm), and that of uranium atoms is 196 pm. The covalent radius of
hydrogen atoms is just 31 pm.[1]
The
invention of
atomic force microscopy has simplified atomic imaging, so it's now common to see neat arrays of atoms, sometimes with highlighted
defects, published in
scientific journals. The following figure, selected more for its
aesthetic appeal than anything else, is representative of the present
state of the art in atomic imaging.
Carbon is a low
atomic weight element, but it's been imaged often because of its utility as its
graphene allotrope.
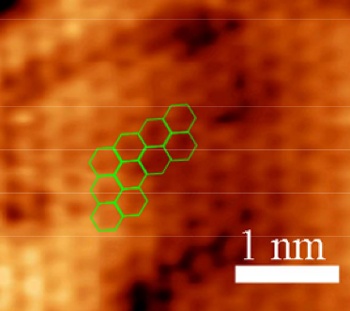
Atomically-resolved scanning tunneling microscope image of graphene on copper.
The bright areas are the regions of highest electron density, where the carbon atoms are located. The planar hexagonal lattice, built from circles of six carbon atoms, is highlighted.
(Fig. S1(a) of ref. 2, via the arXiv Preprint Server.)[2)]
A team of
scientists from the
IBM Zurich Research Laboratory, the
Universidade de Santiago de Compostela (Spain), and the
French National Centre for Scientific Research (CNRS,
Toulouse Cedex, France), have advanced the state of the art in atomic imaging by producing images of bits of graphene that have a resolution high enough to allow measurement of the different
bond lengths that exist from from the center of the graphene patch to its edge (see figure).[3-6]
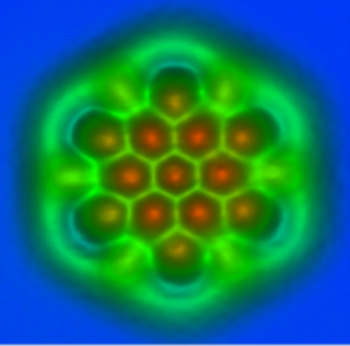
I always thought that carbon atoms were black.
An atomic force micrograph of a small piece of a graphene sheet.
The AFM tip was functionalized with a carbon monoxide molecule.
(IBM Research - Zurich image, used with permission))
The IBM members of this team were the first to image a single
molecule in 2009.[4] The recently produced images have used the technique of frequency modulation atomic force microscopy (FM-AFM) with a functionalized AFM probe tip. The
metal tip held a single
carbon monoxide (CO) molecule at the tip to localize and amplify the signal.[4-5] The apparatus was cooled to -268 °
C, otherwise the
atomic vibrations would have blurred the images.[4]
Said IBM scientist
Leo Gross, who was an author of the paper on this work which made the cover of the September 14, 2012, issue of
Science,[6]
"We found two different contrast mechanisms to distinguish bonds. The first one is based on small differences in the force measured above the bonds. We expected this kind of contrast but it was a challenge to resolve... The second contrast mechanism really came as a surprise: Bonds appeared with different lengths in AFM measurements. With the help of ab initio calculations we found that the tilting of the carbon monoxide molecule at the tip apex is the cause of this contrast."[3]
The functionalized tip allowed a measurement of bond length to a precision of three picometers.[3] This is just 5% of the diameter of a hydrogen atom. The research team is set to investigate the performance of molecules other than CO.[4] I think that
ammonia (NH
3) might be a good candidate. Measurement of interatomic spacing from place to place on a surface could assist research in such areas as
catalysis,
photovoltaics, and
molecular electronics.[5]
Now that we've covered the
scientific aspects of atomic imaging, it's time to take off our
lab coat, put on our
philosopher's fez, and talk about whether or not we really are "seeing" atoms. I wrote an article on this topic many years ago for a general interest
magazine.[7] In that article I used the example of the
oil painting,
The Treachery of Images (La trahison des images, 1928–1929), by
Belgian surrealist artist,
René Magritte.
This is a
copyrighted work of art, presently at the
Los Angeles County Museum of Art, so I can't show its image here; but a low resolution image is
available at Wikipedia. The painting shows a
smoker's pipe and the
inscription, "Ceci n'est pas une pipe" ("This is not a pipe"). Magritte's point being that this is an image of a pipe, and not a pipe.
When you use an
optical microscope to view a small object, you actually do see the object. The microscope acts as a
magnifier for the light that reaches your eyes, but the vision process is the same as always. Atomic force microscopes are not magnifiers of anything that can be seen, so what appears on a
computer display is a representation of atoms. There's no direct linkage to the
human sense of
sight, so we really aren't seeing atoms.
This is not a pipe from the heating system of my house.
(Photo by the author)
![]()
References:
- Covalent Radius 2008, Web Elements.
- Jifa Tian, Helin Cao, Wei Wu, Qingkai Yu and Yong P. Chen, "Direct Imaging of Graphene Edges: Atomic Structure and Electronic Scattering," arXiv Preprint Server, July 25, 2011.
- IBM Scientists First to Distinguish Individual Molecular Bonds, IBM Press Release, September 14, 2012.
- Jason Palmer, "Atomic bond types discernible in single-molecule images," BBC News, September 13, 2012.
- Ruben Perez, "Discriminating Chemical Bonds," Science, vol. 337, no. 6100 (September 14, 2012), pp. 1305-1306.
- L. Gross, F. Mohn, N. Moll, B. Schuler, A. Criado, E. Guitian, D. Pena, A. Gourdon and G. Meyer, "Bond-Order Discrimination by Atomic Force Microscopy," Science, vol. 337, no. 6100 (September 14, 2012), pp. 1326-1329.
- D.M. Gualtieri, Cyborgs and Atomic Microscopes, Phi Kappa Phi Forum, vol. 84, no. 2. pp. 6-7 (Spring 2004).
Permanent Link to this article
Linked Keywords: Atomic theory of matter; 20th century; twentieth century; photograph; photographic; X-ray crystallography; Xray diffraction; atom; crystal structure; heavy element; tungsten; uranium; atomic radius; electron; covalent radius; picometer; pm; hydrogen; invention; atomic force microscopy; defect; scientific journal; aesthetic; state of the art; carbon; relative atomic mass; atomic weight; chemical element; element; graphene; allotrope; copper; electron density; hexagonal tiling; planar hexagonal lattice; arXiv Preprint Server; scientist; IBM Zurich Research Laboratory; Universidade de Santiago de Compostela (Spain); French National Centre for Scientific Research; Toulouse Cedex, France; bond length; carbon monoxide; molecule; metal tip; Celsius; C; atomic vibration; Leo Gross; Science; ammonia; catalysis; photovoltaics; molecular electronics; scientific; white coat; lab coat; philosophy; philosopher; fez; magazine; oil painting; The Treachery of Images; La trahison des images, 1928–1929; Belgian; surrealist artist; René Magritte; copyright; Los Angeles County Museum of Art; Wikipedia; smoking pipe; tobacco; smoker's pipe; inscription; optical microscope; magnification; magnifier; computer display; human sense; sight; pipe; hot water baseboard; heating system.