Vegard and the Periodic Table
January 24, 2012
All
materials scientists are familiar with
Vegard's law. This law simply states that when you combine
elements to form an
alloy, the
lattice constant will follow a
linear trend with the element concentrations, provided that there is no
phase change.[1] I relied on Vegard's law often when I needed to match
epitaxial layers to a particular substrate
crystal. A recent example of Vegard's law appears in the figure.[2]
Vegard is featured in a recent paper by
Helge Kragh, a professor in the
Department of Physics and Astronomy,
Aarhus University (Aarhus, Denmark).[3]
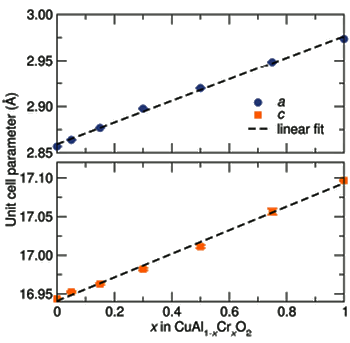
Vegard's law as demonstrated in the lattice constant increase when chromium is substituted for aluminum in CuAlO2.
CuAlO2 is a rhombohedral crystal, so there are two lattice constants that specify its unit cell.
(Fig. 2 of Ref. 2, via the (arXiv Preprint Server). [2)]
Lars Vegard (1880‐1963) was a
Norwegian physicist and
physical chemist who spent most of his working life at the
University of Oslo. He served as vice president of the
International Union of Pure and Applied Physics from 1932-1940. Surprisingly, for materials scientists, Vegard's primary research interest was
spectroscopy of
Earth's aurora. As his eponymous law shows, he worked for a time in
X-ray crystallography. He published
crystal structures of several materials, including
rutile (
titanium dioxide, TiO
2) and
silver, and he published his law in a 1921 paper.[3]
Vegard had an important role in the history of X-ray crystallography beyond Vegard's law. While a
graduate student under
Wilhelm Wien, Vegard was among the first to hear about
Max von Laue's discovery of X‐ray diffraction by crystals. Vegard, who had a
Cambridge University connection since he had spent some time there with
J.J. Thomson in 1907, sent a letter, dated June 26, 1912, to
William Henry Bragg informing him of von Laue's discovery. This was the apparent impetus for the work by Bragg and his son,
William Lawrence Bragg that led to their
Nobel Prize in Physics in 1915. [3]
As Kragh points out, Vegard appears to have been the first to attempt an understanding of the
periodic table in terms of
electron configuration. He developed his ideas and proposed a theory of the elements in a series of papers from 1916-1920. His electron configurations were based on the prevailing
quantum theory of atoms at that time, and they had the feature that the
principal quantum number was related to the shells of Bohr's atomic model.[3] I reviewed Bohr's atomic model in a
recent article (Bohr Model of the Atom, January 3, 2012; see, also, ref. 4).
Of course, the key to understanding the periodic table is in knowing that there are multiple periodicities; that is, knowing that different numbers of electrons will form a closed shell, leaving the remainder for
chemical bonding. Vegard chose the simple model that electrons circled their
nucleus in rings (see figure). The model had the feature that the lower shells with smaller numbers of electrons would have smaller diameters that fit inside the outer shells. It also explained the spectral properties of atoms as electrons jumped from shell to shell.
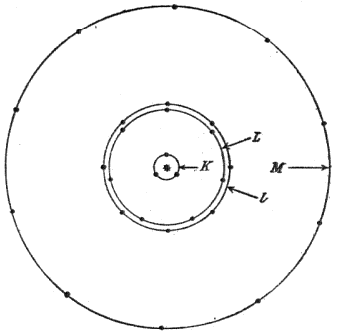
Vegard's atomic model.
This simple model explained the observed atomic spectra and the chemical properties of the elements.
(Fig. 3 of Ref. 3, via the (arXiv Preprint Server). [3)]
As I wrote in the previous article about the Bohr model, there are stability problems with such an arrangement of electrons, so Vegard's model, and other similar models, were replaced by their more familiar successors. Thus, Vegard is known by his law,and not by his atomic theory.
References:
- Materialia Indica Classics in materials science: Vegard's law of linear relationship between lattice parameter and alloy composition, March 14, 2009 by "Guru".
- Phillip T. Barton, Ram Seshadri, Andrea Knöller and Matthew J. Rosseinsky, "Structural and magnetic characterization of the complete delafossite solid solution (CuAlO2){1-x}(CuCrO2){x}," arXiv Preprint Server, October 5, 2011.
- Helge Kragh, "An early explanation of the periodic table: Lars Vegard and X-ray spectroscopy," arXiv Preprint Server, December 16, 2011.
- Helge Kragh and Kristian Hvidtfelt Nielsen, "Spreading the gospel: The Bohr atom popularised," arXiv Preprint Server, December 12, 2011.
- Professor Lars Vegard (3/2 1880 – 21/12 1963), The Auroral Researcher, Physics Department, National University of Ireland Web Site.
Permanent Link to this article
Linked Keywords: Materials science; materials scientist; Vegard's law; element; alloy; lattice constant; linear trend; phase change; epitaxial layer; crystal; Helge Kragh; Department of Physics and Astronomy; Aarhus University (Aarhus, Denmark); chromium; aluminum; rhombohedral; unit cell; arXiv; Lars Vegard; Norwegian; physicist; physical chemist; University of Oslo; International Union of Pure and Applied Physics; spectroscopy; Earth's aurora; X-ray crystallography; crystal structure; rutile; titanium dioxide; silver; graduate student; Wilhelm Wien; Max von Laue; Cambridge University; J.J. Thomson; William Henry Bragg; William Lawrence Bragg; Nobel Prize in Physics; periodic table; electron configuration; quantum theory of atoms; principal quantum number; chemical bonding; nucleus.