The Periodic Table Table
April 17, 2012
When I was young, and interested in all aspects of science, I had a collection of
chemical elements on my book shelf. Most elements, at least at that time, were not that hard to find in their pure forms. Nowadays, many of these are handled as
toxic materials, so today's youngsters could never amass the collection that I had. For example, I had a small drop of
mercury in a
glass vial, compliments of our family
dentist, who advised me not to drink it. I doubt that something like that could happen today.
Along with that drop of mercury, I had
carbon from a
pencil,
aluminum from
foil,
neon in a
neon bulb,
lead,
tin,
chromium (a
plated tube),
iron (a
nail);
nickel,
copper and
silver (
coins);
zinc (from a
battery),
tungsten, and probably
thorium, from a
light bulb; and
gold (a small piece of
jewelry). Those were the days of
$35/troy ounce gold, never to be seen again.
After the pure elements, I scored a few more elements via their compounds. These were
sodium,
chlorine and
iodine from
table salt;
magnesium and
sulfur from
Epsom salt (magnesium sulfate),
boron from
borax (sodium tetraborate),
calcium from
gypsum (calcium sulfate dihydrate, from
drywall wallboard) and from
chalk (
calcium carbonate, CaCO
3);
phosphorus from
laundry detergents, and
silicon from
sand (SiO
2).
In my professional career I've worked with quite a few of the remaining elements, including many of the
rare earth elements that most other
scientists don't encounter. By my count, I've used 76 of the elements in various studies. Most of the remaining 42 elements are unstable, and they undergo
radioactive decay into other elements.
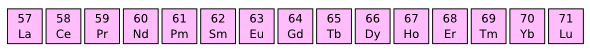
The Lanthanide elements were my main preoccupation for many years when I was developing magnetic materials. I synthesized materials using all these elements, except promethium (Pm). (Via Wikimedia Commons))
My youthful
element collecting pales in comparison to that of
Theodore Gray, one of the founders of
Wolfram Research. Gray, who is presently Director of User Interface Technology at Wolfram, has built an actual
table with compartments containing specimens of nearly all the elements. His table has been featured in various articles for more than a decade.

Theo Gray, creator of the Periodic Table Table, seated at his table.
Some specimens of elements can be seen on the shelves behind him.
(Screen capture from a YouTube video). [2)]
Gray was awarded an
Ig Nobel Prize in Chemistry in 2002, and he was the recipient of the 2011
James T. Grady-James H. Stack Award for Interpreting Chemistry for the Public of the
American Chemical Society.
The Periodic Table Table is not Gray's only contribution to public awareness of
chemistry. Gray also publishes a regular series in
Popular Science entitled, "
Gray Matter," which was nominated for a 2010
National Magazine Award for Best Column.
Articles from "Gray Matter" were collected into a book in 2009 entitled, "Mad Science: Experiments You Can do at Home—But Probably Shouldn't."[4] His web site includes a wide variety of
periodic table products for sale.
Shown below is the
Dysprosium compartment of the Periodic Table Table. Dysprosium used to form the highly
magnetostrictive alloy,
Terfenol-D Tb
0.3Dy
0.7Fe
2.

Dysprosium compartment of the Periodic Table Table
(Screen capture from a YouTube video). [2)]
![]()
References:
- Kirk Zamieroski, "The Periodic Table Table Featuring Theo Gray," Byte Size Science, February 22, 2012.
- The Periodic Table Table Featuring Theo Gray, YouTube video by BytesizeScience, February 22, 2012.
- Michael Bernstein and Michael Woods, "New American Chemical Society video showcases the "Periodic Table Table'," American Chemical Society Press Release, March 1, 2012.
- Theodore Gray, "Theo Gray's Mad Science: Experiments You Can do At Home - But Probably Shouldn't," Black Dog & Leventhal Publishers, May 25, 2011, 240 pp. (via Amazon)
- Theodore Gray Website.
Permanent Link to this article
Linked Keywords: Chemical element; toxic material; mercury; glass vial; dentist; carbon; pencil; aluminum; foil; neon; neon bulb; lead; tin; chromium; plated; iron; nail; nickel; copper; silver; coin; zinc; zinc-carbon battery; battery; tungsten; thorium; incandescent light bulb; light bulb; gold; jewelry; gold standard; $35/troy ounce gold; sodium; chlorine; iodine; iodised salt; table salt; magnesium; sulfur; magnesium sulfate; Epsom salt; boron; borax; calcium; gypsum; drywall wallboard; chalk; calcium carbonate; phosphorus; laundry detergent; silicon; sand; rare earth element; scientist; radioactive decay; Lanthanide element; magnetic material; promethium; Wikimedia Commons; element collecting; Theodore Gray; Wolfram Research; table; YouTube video; Ig Nobel Prize in Chemistry; James T. Grady-James H. Stack Award for Interpreting Chemistry for the Public; American Chemical Society; chemistry; Popular Science; Gray Matter; National Magazine Award for Best Column; periodic table products; Dysprosium; magnetostriction; magnetostrictive; Terfenol-D; Theo Gray's Mad Science: Experiments You Can do At Home - But Probably Shouldn't.