Catalytic Abiogenesis
January 26, 2011
The ancients had curious beliefs about
living things up to the time of
Francis Bacon (1561-1626), when the
scientific method started to develop. It's rather obvious that life springs from other life, but there was always a belief that life could spring from
inorganic matter. In a
Greek myth retold by
Ovid in his
Metamorphoses,[1] after
Zeus destroyed
Earth by a flood (
sounds familiar?),
Deucalion and
Pyrrha were instructed by the Goddess
Themis to repopulate the Earth by throwing the bones of their mother over their shoulder. Their mother was the Earth, her bones were stones, and the stones became people. As if to explain the 50:50 ratio of men and women in the world, Deucalion's stones became men, and Pyrrha's stones became women.
The relationship between life and inanimate material was researched extensively in the seventeenth century.
Jan Baptist van Helmont (1580–1644) did a famous experiment in which he grew a
willow tree in a pot for five years and showed that the weight of the
soil was essentially unchanged. His conclusion was that
water was transformed into tree. This same van Helmont published a recipe for
spontaneous generation of
mice from husks of
wheat wrapped in old clothing. The scientific method was not developed enough at the time for him to realize that his cloth-wrapped wheat should have been placed in a cage. In van Helmont's time, such spontaneous generation of life was considered to be frequent and common, and it was only by the time of
Louis Pasteur that the "
germ theory" developed and
cellular processes were understood.
At some point in the history of the Earth, self-replicating living matter appeared and took over the surface. That process where life originally forms from non-living matter is called
abiogenesis. It's always been an interesting question, but it's gotten a lot more attention lately when applied universally; that is, to possible formation of life on other planets. That's a part of the recent discipline of
astrobiology.
About the time I was just getting interested in science,
Stanley Miller, who was working towards his
Ph.D. in
Chemistry under
Nobelist,
Harold Urey, performed an interesting experiment at the
University of Chicago. He placed
ammonia,
methane,
hydrogen and
water, constituents that were thought to be present in the
reducing atmosphere of the early Earth, in a closed reaction vessel and subjected them to artificial
lightning in the form of
electrical discharges. The reaction produced
amino acids, building blocks for living things. I remember reading about Miller's experiment when I was a child, since there were many articles about it in the popular press.
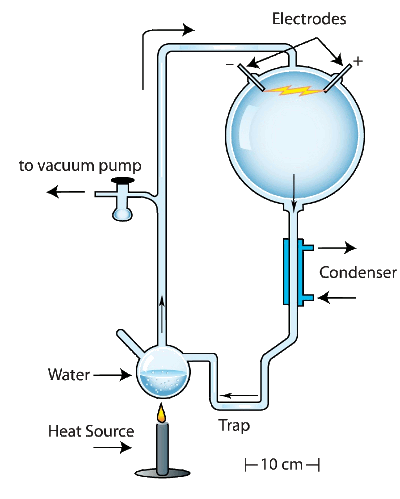
Miller Experiment,
Illustration
by Ned Shaw,
Indiana University
(modified)
Miller's experiment is so interesting that when a graduate student recently discovered sealed glass vials that contained samples from Miller's original experiment, there was a
NASA-funded effort to do a reanalysis of the reaction products with much more sensitive modern equipment.[2] As it turned out, an experiment that Miller didn't publish, one that simulated conditions near erupting
volcanoes, was found by modern equipment to have produced twenty two amino acids. The evidence from this experiment is especially important, since our idea of Earth's early atmosphere has changed since Miller's time. Scientists now think that it contained mostly
carbon dioxide,
carbon monoxide, and
nitrogen; but hydrogen and methane could have come from volcanic sources. Volcanic eruptions produce lightning, also, as well as
carbonyl sulfide gas, which can link amino acids into
peptides.
As it turns out, creation of amino acids is so easy, it's been done in an
asteroid that made a recent terrestrial impact.[3-5] One of the discoverers of this was
Daniel P. Glavin of the Astrobiology Analytical Laboratory at NASA’s
Goddard Space Flight Center. Glavin was a member of the team that did the reanalysis of Miller's samples. Glavin is a
physicist and
earth scientist who now specializes in extraction and analysis of extraterrestrial amino acids in
meteorites. Meteorites, remnants of
2008 TC3, a small
carbonaceous asteroid about 6-15 feet in dimension created when two asteroids collided in the past, impacted the
Nubian Desert of northern
Sudan on October 7, 2008. They were collected by
Peter Jenniskens of NASA and the
SETI Institute and Muawia Shaddad of the
University of Khartoum.[6]

A remnant of the meteorite known as 'Almahata Sitta' associated with asteroid 2008 TC3. Photo by Peter Jenniskens
The asteroid remnants turned out to be the first
Ureilite meteorite to be found in pristine condition. Ureilites are a rare type of meteorite thought to have formed in the
solar nebula. The collision, which is supposed to have generated temperatures of more than a thousand degrees centigrade because of the presence of certain
minerals that form only at such temperatures, should have destroyed all organic material that may have existed in either of the parent asteroids. Analysis of the meteorite fragments showed the presence of 19 different amino acids, some at a concentration of 149 parts per billion. The analysis team is certain that this is not terrestrial contamination, since the acids are in a
racemic mixture. Terrestrial organisms produce just
left-handed amino acids.
It appears that the amino acids formed from gaseous elements as they cooled. Galvin speculates that grains of
nickel or
iron in the asteroid could have acted as a
catalyst that aided amino acid formation as carbon monoxide, molecular hydrogen and ammonia gases cooled below about 500
oC.[5] Galvin is the principal author of a paper that describes this research in the October/November 2010 issue of Meteoritics and Planetary Science.[7]
References:
- Ovid, "Metamorphoses," Book I, ll. 381-384; English translation.
Mota dea est sortemque dedit: 'discedite templo et velate caput cinctasque resolvite vestes ossaque post tergum magnae iactate parentis!'
The Goddess was moved, and gave this response: 'Depart from my temple, cover your heads, loosen your garments, and throw behind your backs the bones of your great mother!'
- David Bricker, Annie Reisewitz, Daniella Scalice, Nancy Neal-Jones and Bill Steigerwald, "Volcanoes May Have Provided Sparks and Chemistry for First Life," NASA Goddard Space Flight Center, October 16, 2008.
- Nancy Neal-Jones and Bill Steigerwald, "Building Blocks of Life Created in "Impossible" Place," Goddard Press Release No. 10-111, December 15, 2010.
- More than One Way to Make Amino Acids, Astrobio.net, December 17, 2010.
- Ron Cowen, "Space rock surprise-Amino acids in meteorite may have unexpected origin," Science News, December 21, 2010.
- Roberta Kwok, "Astronomy: The rock that fell to Earth," Nature, vol.458, no. 7237 (March 26, 2009), pp. 401-403.
- Daniel P. Glavin, Andrew D. Aubrey, Michael P. Callahan, Jason P. Dworkin, Jamie E. Elsila, Eric T. Parker, Jeffrey L. Bada, Peter Jenniskens and Muawia H. Shaddad, "Extraterrestrial amino acids in the Almahata Sitta meteorite," Meteoritics & Planetary Science, vol. 45, no. 10-11 (October/November 2010), pp. 1695-1709.
- D.M. Gualtieri, "Trace Elements and the Panspermia Hypotheses," Icarus, vol. 30, no. 1 (January, 1977) pp. 234-238.
Permanent Link to this article
Linked Keywords: Life; Francis Bacon; scientific method; inorganic_compound; Greek_mythology; Greek myth; Ovid; Metamorphoses; Zeus; Earth; Noah; Deucalion; Pyrrha; Themis; Jan Baptist van Helmont; willow tree; soil; water; spontaneous generation; mouse; mice; wheat; Louis Pasteur; germ theory; cell; cellular; abiogenesis; astrobiology; Stanley Miller; Doctor of Philosophy; Ph.D.; Chemistry; Nobel Prize; Harold Urey; University of Chicago; ammonia; methane; hydrogen; water; reducing atmosphere; lightning; electrical discharge; amino acid; Miller Experiment; Indiana University; NASA; volcano; carbon dioxide; carbon monoxide; nitrogen; carbonyl sulfide; peptide; asteroid; Daniel P. Glavin; Goddard Space Flight Center; physicist; earth scientist; meteorite; 2008 TC3; carbonaceous; Nubian Desert; Sudan; Peter Jenniskens; SETI Institute; University of Khartoum; Almahata Sitta; Ureilite meteorite; solar nebula; mineral; racemic mixture; levorotation; dextrorotation; nickel; iron; catalyst; Ovid, "Metamorphoses," Book I, ll. 381-384.