
Making Diamonds
December 16, 2013 Contrary to advertising claims, diamonds are not forever. Diamond will burn, just like its chemical cousin, charcoal, if combustion is initiated. The combustion product is carbon dioxide. Thermodynamics confirms this observation, since the free energy of the combustion reaction at standard temperature and pressure (298.15 K and 1 atmosphere) is negative;[1] viz.,C(Diamond) + O2(gas) -> CO2(gas)Although the reaction is highly favored at room temperature, this combustion reaction has an activation energy that must be overcome before it happens. As a consequence, diamond will only ignite at a temperature of 850-1,000°C in air, or 720-800°C in pure oxygen. The combustion of diamond to form carbon dioxide was experimentally confirmed by Antoine Lavoisier in 1772. Lavoisier concentrated the Sun's rays with a lens to heat a diamond in an oxygen atmosphere to produce carbon dioxide. Later, the chemist, Smithson Tennant, showed that the combustion of graphite produced the same result, confirming that diamond and graphite are allotropic forms of carbon.
ΔGf(Diamond) = 0.693 kcal/mole
ΔGf(Oxygen) = 0 kcal/mole
ΔGf(Carbon Dioxide) = -94.258 kcal/mole
ΔG(Reaction) = -94.951 kcal/mole
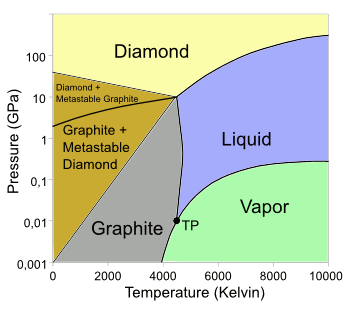 | The carbon phase diagram. The triple point (TP) is around 4600 K and 10.8 MPa. (Wikimedia Commons image, from data in ref. 2, modified using Inkscape.)[2] |
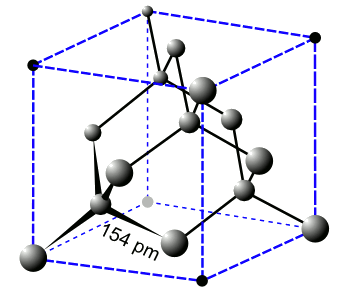 | The crystal structure of diamond is cubic, with each carbon atom joined to others by a 154 picometer bond. (Wikimedia Commons image, modified using Inkscape.) |
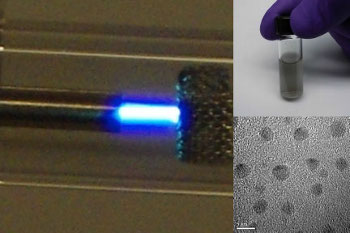 | Nanodiamond synthesis at Case Western Reserve University. Bright blue plasma is shown at the left, producing nanodiamonds at bottom. (Case Western Reserve University images.[7]) |
"This is not a complex process: ethanol vapor at room temperature and pressure is converted to diamond... We flow the gas through a plasma, add hydrogen and out come diamond nanoparticles. We can put this together and make them in almost any lab."[8]One helpful factor was the surface energy of diamond. This makes the nanodiamonds more stable than graphite, since nanoparticles have a high ratio of surface area to volume. This ensured that most nucleated nanoparticles would be diamond, and the hydrogen removed any non-diamond particles.[8]
References:
- Free energy data from L. B. Pankratz, "Thermodynamic Properties of Elements and Oxides," U. S. Bureau of Mines Bulletin 672, U. S. Government Printing Office (1982).
- J.M. Zazula, "On Graphite Transformations at High Temperature and Pressure Induced by Absorption of the LHC Beam," LHC Project Note 78, CERN, January 18, 1997 (PDF File).
- Artificial Diamonds, Human Touch of Chemistry Web Site.
- Howard Tracy Hall, "Diamond synthesis," U.S. Patent No. 2,947,608, August 2, 1960 (via Google Patents).
- H. P. Bovenkerk, F. P. Bundy, H. T. Hall, H. M. Strong and R. H. Wentorf, Jr., "Preparation of Diamond," Nature, vol. 184 (October 10, 1959), pp. 1094-1098 (PDF reprint at Tracy Hall Web Site).
- Thomas H. Maugh II, "General Electric chemist invented process for making diamonds in lab - H. Tracy Hall, 1919 - 2008," Los Angeles Times, July 31, 2008.
- Ajay Kumar, Pin Ann Lin, Albert Xue, Boyi Hao, Yoke Khin Yap and R. Mohan Sankaran, "Formation of nanodiamonds at near-ambient conditions via microplasma dissociation of ethanol vapour," Nature Communications, vol. 4, article no. 2618 (October 21, 2013), doi:10.1038/ncomms3618.
- Researchers develop way to inexpensively create nanodiamonds in lab setting, Case Western Reserve University Daily, October 28, 2013.
- Artificial Diamonds, Human Touch of Chemistry Web Site.