
Supercooled Water
December 22, 2011 I've used chemical thermodynamics very often to predict what will happen when materials are combined at a given temperature. When I presented my results, I was always careful to point out that thermodynamics gives answers for systems in equilibrium, and the kinetics of a reaction are another thing altogether. You only have to mix hydrogen and oxygen together to see that this is the case. Nothing happens, unless an electrical spark or a catalytic surface is introduced into the system. When that happens, you're quite convinced that the predicted reaction takes place. Water has many unusual properties, so many that there's a web site dedicated to them.[1] These unusual properties are likely the result of water being small molecule, and the forces holding these molecules together in a liquid or solid coming from hydrogen bonding. There are sixteen solid phases of water ice, named ice Ih (our "normal" ice) to ice XV, existing at different temperatures and pressures, as shown in the figure.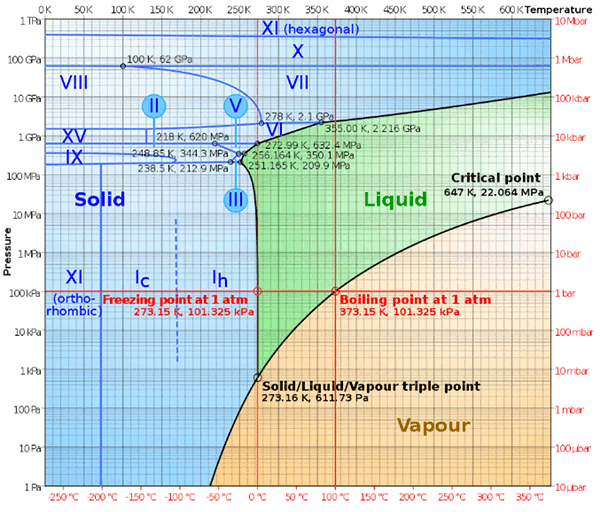 Temperature-pressure phase diagram of water. Ice IV and Ice XII are metastable, so they are missing from this equilibrium diagram. (Image by Cmglee, via Wikimedia Commons).
It's well-known that liquids can be supercooled; that is, they can be cooled below their freezing points without solidifying. Water can be supercooled, so a fair scientific question is how far can water be supercooled, and what are the factors that govern the degree of supercooling. These questions were addressed in a recent study by Valeria Molinero, an assistant professor of chemistry at the University of Utah (Salt Lake City, Utah), and her doctoral student, Emily B. Moore.[2-3]
The properties of supercooled water have been investigated experimentally, but only at the lowest temperature of -42°F (232 K). This is the homogeneous nucleation temperature. Below this temperature, crystallization of water is too rapid for the properties of the remaining liquid to be measured.[3] For this reason, the Utah chemists used computers at the University of Utah's Center for High Performance Computing instead of test tubes.[3] Supercooled water was both simulated at the molecular level and modeled using experimental data.[3]
An important first step in their research was optimization of the computer codes used in such modeling and simulation. They were able to speed computations by a factor of 200, but computation still took thousands of hours of computer time on their simulation of freezing of a 32,768 molecule cluster. The number 32,768, of course, is 215.
They calculated the heat capacity, density and compressibility of water as it is supercooled; and they also simulated the rate of ice crystallization.[3] Their calculations extended to -55°F, the temperature at which the maximum crystallization rate is obtained, and also the lowest possible supercooled temperature.[3]
Temperature-pressure phase diagram of water. Ice IV and Ice XII are metastable, so they are missing from this equilibrium diagram. (Image by Cmglee, via Wikimedia Commons).
It's well-known that liquids can be supercooled; that is, they can be cooled below their freezing points without solidifying. Water can be supercooled, so a fair scientific question is how far can water be supercooled, and what are the factors that govern the degree of supercooling. These questions were addressed in a recent study by Valeria Molinero, an assistant professor of chemistry at the University of Utah (Salt Lake City, Utah), and her doctoral student, Emily B. Moore.[2-3]
The properties of supercooled water have been investigated experimentally, but only at the lowest temperature of -42°F (232 K). This is the homogeneous nucleation temperature. Below this temperature, crystallization of water is too rapid for the properties of the remaining liquid to be measured.[3] For this reason, the Utah chemists used computers at the University of Utah's Center for High Performance Computing instead of test tubes.[3] Supercooled water was both simulated at the molecular level and modeled using experimental data.[3]
An important first step in their research was optimization of the computer codes used in such modeling and simulation. They were able to speed computations by a factor of 200, but computation still took thousands of hours of computer time on their simulation of freezing of a 32,768 molecule cluster. The number 32,768, of course, is 215.
They calculated the heat capacity, density and compressibility of water as it is supercooled; and they also simulated the rate of ice crystallization.[3] Their calculations extended to -55°F, the temperature at which the maximum crystallization rate is obtained, and also the lowest possible supercooled temperature.[3]
 | Molecular simulation of water crystallization from supercooled water. Regular ice is shown in red, and "intermediate ice" is shown in red. (University of Utah image). |
References:
- Martin Chaplin, "Anomalous properties of water," London South Bank University Web Site.
- Emily B. Moore and Valeria Molinero, "Structural transformation in supercooled water controls the crystallization rate of ice," Nature, vol. 479, no. 7374 (November 24, 2011), pp. 506-508.
- Lee J. Siegel, "Supercool - Utah Chemists: Water Doesn't Have to Freeze until -55 F," University of Utah Press Release, November 23, 2011.
- Emily B. Moore and Valeria Molinero, "Structural transformation in supercooled water controls the crystallization rate of ice," Nature, vol. 479, no. 7374 (November 24, 2011), pp. 506-508.