
William Lipscomb
April 18, 2011 When I was a graduate student, there was a cartoon posted on one of the chemistry bulletin boards. It was entitled, "A Boron Chemist's Version of the Periodic Table." It was a Periodic Table, but it was drawn so that the boron square filled as much area as the rest of the table. As a putative metallurgist, I didn't see what was that interesting about boron, except for the fact that the chemistry building was once evacuated because of a leaky boron trifluoride BF3 gas cylinder. All the action seemed to be at carbon, boron's next door neighbor. William Nunn Lipscomb, an important figure in boron chemistry, died on April 14, 2011, at age 91.[1-4] | William Nunn Lipscomb, circa 1980. Photo by James S. Lipscomb, via Wikimedia Commons |
The subsequent low temperature studies of single crystals of these volatile and unstable boranes were not without hazards. Vacuum line techniques were learned as we needed them. Fortunately, no serious injuries were incurred as a result of several explosions resulting from cracks in these vacuum systems. I was relieved, on one occasion, when I had taken Russell Grimes to a hospital in Cambridge after one of these explosions to hear the doctor tell me, "Louis Fieser sends me much more interesting cases than you do."Fieser was a Harvard organic chemist who was involved with a wartime project to have bats with timed incendiary charges start fires in Japan. During World War II, Lipscomb did military research during the day and his graduate research at night. Lipscomb's doctoral dissertation was locked in a safe for several years because it contained classified research.[2] In order to elucidate the bonding structures of the boranes, Lipscomb and his students would crystallize them at low temperature and perform X-ray crystallographic analysis. One early, and quite unusual discovery, was that the boron atoms in some boranes were bonded together by a hydrogen atom. Until that time, bonds were considered to be possible only when atoms shared an electron pair. His seminal paper, "The Valence Structure of the Boron Hydrides," coauthored with Bryce Crawford Jr. and W. H. Eberhardt, appeared in 1954.[6] Even Pauling was impressed.[7]
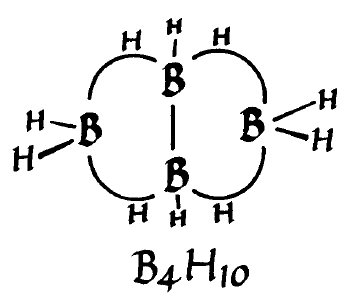 | Drawing of the structure of B4H10 from William Lipscomb's Nobel Prize lecture. (Nobel Prize Foundation) |
References:
- William Lipscomb dies at 91 - Harvard professor won Nobel Prize in chemistry, Campus & Community Obituaries, Harvard University Web Site, April 15, 2011.
- By Thomas H. Maugh II, "William N. Lipscomb dies at 91; won Nobel Prize in chemistry," Los Angeles Times, April 16, 2011.
- Mark Feeney, "Nobel laureate and Harvard professor dead at 91 - William N. Lipscomb researched boranes," The Boston Globe, April 16, 2011
- William Lipscomb page on the Nobel Prize Web Site.
- Nobel Prize Lecture by William Lipscomb, Nobel Prize Web Site, 1976.
- W. H. Eberhardt, B. Crawford, Jr. and W. N. Lipscomb, "The Valence Structure of the Boron Hydrides," J. Chem. Phys. vol. 22, no. 6 (June 1, 1954), pp. 989-1001.
- Linus Pauling, Letter to Crawford, Eberhardt, Lipscomb, OSU Special Collections, March 30, 1954.
- Interview with Professor William N. Lipscomb by Joanna Rose, Nobel Prize Web Site, December 3, 2001.
- By Thomas H. Maugh II, "William N. Lipscomb dies at 91; won Nobel Prize in chemistry," Los Angeles Times, April 16, 2011.